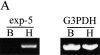Exportin-5, a novel karyopherin, mediates nuclear export of double-stranded RNA binding proteins - PubMed (original) (raw)
Exportin-5, a novel karyopherin, mediates nuclear export of double-stranded RNA binding proteins
Amy M Brownawell et al. J Cell Biol. 2002.
Abstract
We have identified a novel human karyopherin (Kap) beta family member that is related to human Crm1 and the Saccharomyces cerevisiae protein, Msn5p/Kap142p. Like other known transport receptors, this Kap binds specifically to RanGTP, interacts with nucleoporins, and shuttles between the nuclear and cytoplasmic compartments. We report that interleukin enhancer binding factor (ILF)3, a double-stranded RNA binding protein, associates with this Kap in a RanGTP-dependent manner and that its double-stranded RNA binding domain (dsRBD) is the limiting sequence required for this interaction. Importantly, the Kap interacts with dsRBDs found in several other proteins and binding is blocked by double-stranded RNA. We find that the dsRBD of ILF3 functions as a novel nuclear export sequence (NES) in intact cells, and its ability to serve as an NES is dependent on the expression of the Kap. In digitonin-permeabilized cells, the Kap but not Crm1 stimulated nuclear export of ILF3. Based on the ability of this Kap to mediate the export of dsRNA binding proteins, we named the protein exportin-5. We propose that exportin-5 is not an RNA export factor but instead participates in the regulated translocation of dsRBD proteins to the cytoplasm where they interact with target mRNAs.
Figures
Figure 1.
Exportin-5 is a Kapβ family member. (A) Amino acid sequences of exportin-5, Crm1, and the S. cerevisiae Kapβ, Msn5p, were aligned using CLUSTAL. Identical residues are shown in black; closely related residues are shown in gray. (B) Unrooted phylogenetic tree of exportin-5 and the 14 S. cerevisiae Kapβs generated using the ProtPars program in the Phylip package. A total of 1,000 boot-strapped replica datasets were run from an alignment of the S. cerevisiae Kap and exportin-5 sequences that was generated using Clustal W. Each dataset was analyzed 13 times with the sequence input order randomized each time. The tree represents the consensus of all trees produced. Values on the branches refer to the proportion of times a given branch was recorded among all trees. Values >90% indicate strong support, values 90–60% indicate moderate to weak support, and values <60% indicate no support for a given branching pattern. (C) Northern blotting of mRNAs from human tissues was performed with a probe generated using the exportin-5 cDNA as a template. The same blot was hybridized with a β-actin probe as a loading control. The exportin-5 sequence data are available from GenBank/EMBL/DDBJ under accession no. AF298880.
Figure 2.
Exportin-5 interacts with the RanGTPase and nucleoporins. (A) HF7c (MATa) yeast that express the indicated bait proteins as GAL4 DBD fusions were mated with the W303 (MATα) strain expressing the VP16 transactivation domain either alone (VP16) or as a fusion with Ran (VP16-Ran). Diploid yeast were selected on Leu−Trp− plates and replica plated onto Leu−Trp−His− ±3-aminotriazole (3AT) as indicated. (B) Exportin-5–His6 was incubated with either GST-RanGDP or GST-RanGTP bound to GSH beads. Bound proteins were separated by SDS-PAGE and detected by Coomassie staining. (C) The apparent dissociation constant of the RanGTP–exportin-5 complex was estimated using a RanGAP protection assay. Ran[γ-32P]GTP was preincubated for 15 min with the indicated concentrations of exportin-5–His6. The GTPase reaction was started by addition of RanGAP. After 3 min, the proteins were filter bound and washed. Hydrolysis of GTP was determined by the amount of Ran[γ-32P]GTP bound. Assays were in duplicate and are representative of two independent experiments. Error is expressed as the differences from the means. (D) HA–importin β and HA–exportin-5 were immunoprecipitated from HEK293 cell lysates to which RanQ69L was added as indicated. Untransfected HEK293 cells were used as a control for nonspecific nucleoporin binding (Mock IP). Lysate was prepared by solubilizing HEK293 cells with boiling sample buffer. Coimmunoprecipitated nucleoporins were detected with mAb RL1 (Snow et al., 1987). RL1 efficiently detected RanBP2, Can/Nup214, and Nup153 but not other nucleoporins in HEK293 lysate. HA–importin β and HA–exportin-5 were detected using mAb 12CA5. A protein not characterized previously as reacting with the RL1 mAb bound exportin-5 in the absence of RanQ69L and is denoted with an asterisk. (E) Heterotypic cell fusions were performed between BHK21 cells, transiently transfected with pKmyc–exportin-5, and a stably transfected HeLa cell line expressing GFP–streptavidin–NLS (GSN2). After fusion with polyethylene glycol, the cells were fixed and stained for myc-tagged proteins with 9E10 and a Texas red–conjugated secondary antibody. Nuclei were visualized using DAPI. The arrows denote the GSN2 cells in the fusion. Bar, 10 μm.
Figure 2.
Exportin-5 interacts with the RanGTPase and nucleoporins. (A) HF7c (MATa) yeast that express the indicated bait proteins as GAL4 DBD fusions were mated with the W303 (MATα) strain expressing the VP16 transactivation domain either alone (VP16) or as a fusion with Ran (VP16-Ran). Diploid yeast were selected on Leu−Trp− plates and replica plated onto Leu−Trp−His− ±3-aminotriazole (3AT) as indicated. (B) Exportin-5–His6 was incubated with either GST-RanGDP or GST-RanGTP bound to GSH beads. Bound proteins were separated by SDS-PAGE and detected by Coomassie staining. (C) The apparent dissociation constant of the RanGTP–exportin-5 complex was estimated using a RanGAP protection assay. Ran[γ-32P]GTP was preincubated for 15 min with the indicated concentrations of exportin-5–His6. The GTPase reaction was started by addition of RanGAP. After 3 min, the proteins were filter bound and washed. Hydrolysis of GTP was determined by the amount of Ran[γ-32P]GTP bound. Assays were in duplicate and are representative of two independent experiments. Error is expressed as the differences from the means. (D) HA–importin β and HA–exportin-5 were immunoprecipitated from HEK293 cell lysates to which RanQ69L was added as indicated. Untransfected HEK293 cells were used as a control for nonspecific nucleoporin binding (Mock IP). Lysate was prepared by solubilizing HEK293 cells with boiling sample buffer. Coimmunoprecipitated nucleoporins were detected with mAb RL1 (Snow et al., 1987). RL1 efficiently detected RanBP2, Can/Nup214, and Nup153 but not other nucleoporins in HEK293 lysate. HA–importin β and HA–exportin-5 were detected using mAb 12CA5. A protein not characterized previously as reacting with the RL1 mAb bound exportin-5 in the absence of RanQ69L and is denoted with an asterisk. (E) Heterotypic cell fusions were performed between BHK21 cells, transiently transfected with pKmyc–exportin-5, and a stably transfected HeLa cell line expressing GFP–streptavidin–NLS (GSN2). After fusion with polyethylene glycol, the cells were fixed and stained for myc-tagged proteins with 9E10 and a Texas red–conjugated secondary antibody. Nuclei were visualized using DAPI. The arrows denote the GSN2 cells in the fusion. Bar, 10 μm.
Figure 2.
Exportin-5 interacts with the RanGTPase and nucleoporins. (A) HF7c (MATa) yeast that express the indicated bait proteins as GAL4 DBD fusions were mated with the W303 (MATα) strain expressing the VP16 transactivation domain either alone (VP16) or as a fusion with Ran (VP16-Ran). Diploid yeast were selected on Leu−Trp− plates and replica plated onto Leu−Trp−His− ±3-aminotriazole (3AT) as indicated. (B) Exportin-5–His6 was incubated with either GST-RanGDP or GST-RanGTP bound to GSH beads. Bound proteins were separated by SDS-PAGE and detected by Coomassie staining. (C) The apparent dissociation constant of the RanGTP–exportin-5 complex was estimated using a RanGAP protection assay. Ran[γ-32P]GTP was preincubated for 15 min with the indicated concentrations of exportin-5–His6. The GTPase reaction was started by addition of RanGAP. After 3 min, the proteins were filter bound and washed. Hydrolysis of GTP was determined by the amount of Ran[γ-32P]GTP bound. Assays were in duplicate and are representative of two independent experiments. Error is expressed as the differences from the means. (D) HA–importin β and HA–exportin-5 were immunoprecipitated from HEK293 cell lysates to which RanQ69L was added as indicated. Untransfected HEK293 cells were used as a control for nonspecific nucleoporin binding (Mock IP). Lysate was prepared by solubilizing HEK293 cells with boiling sample buffer. Coimmunoprecipitated nucleoporins were detected with mAb RL1 (Snow et al., 1987). RL1 efficiently detected RanBP2, Can/Nup214, and Nup153 but not other nucleoporins in HEK293 lysate. HA–importin β and HA–exportin-5 were detected using mAb 12CA5. A protein not characterized previously as reacting with the RL1 mAb bound exportin-5 in the absence of RanQ69L and is denoted with an asterisk. (E) Heterotypic cell fusions were performed between BHK21 cells, transiently transfected with pKmyc–exportin-5, and a stably transfected HeLa cell line expressing GFP–streptavidin–NLS (GSN2). After fusion with polyethylene glycol, the cells were fixed and stained for myc-tagged proteins with 9E10 and a Texas red–conjugated secondary antibody. Nuclei were visualized using DAPI. The arrows denote the GSN2 cells in the fusion. Bar, 10 μm.
Figure 2.
Exportin-5 interacts with the RanGTPase and nucleoporins. (A) HF7c (MATa) yeast that express the indicated bait proteins as GAL4 DBD fusions were mated with the W303 (MATα) strain expressing the VP16 transactivation domain either alone (VP16) or as a fusion with Ran (VP16-Ran). Diploid yeast were selected on Leu−Trp− plates and replica plated onto Leu−Trp−His− ±3-aminotriazole (3AT) as indicated. (B) Exportin-5–His6 was incubated with either GST-RanGDP or GST-RanGTP bound to GSH beads. Bound proteins were separated by SDS-PAGE and detected by Coomassie staining. (C) The apparent dissociation constant of the RanGTP–exportin-5 complex was estimated using a RanGAP protection assay. Ran[γ-32P]GTP was preincubated for 15 min with the indicated concentrations of exportin-5–His6. The GTPase reaction was started by addition of RanGAP. After 3 min, the proteins were filter bound and washed. Hydrolysis of GTP was determined by the amount of Ran[γ-32P]GTP bound. Assays were in duplicate and are representative of two independent experiments. Error is expressed as the differences from the means. (D) HA–importin β and HA–exportin-5 were immunoprecipitated from HEK293 cell lysates to which RanQ69L was added as indicated. Untransfected HEK293 cells were used as a control for nonspecific nucleoporin binding (Mock IP). Lysate was prepared by solubilizing HEK293 cells with boiling sample buffer. Coimmunoprecipitated nucleoporins were detected with mAb RL1 (Snow et al., 1987). RL1 efficiently detected RanBP2, Can/Nup214, and Nup153 but not other nucleoporins in HEK293 lysate. HA–importin β and HA–exportin-5 were detected using mAb 12CA5. A protein not characterized previously as reacting with the RL1 mAb bound exportin-5 in the absence of RanQ69L and is denoted with an asterisk. (E) Heterotypic cell fusions were performed between BHK21 cells, transiently transfected with pKmyc–exportin-5, and a stably transfected HeLa cell line expressing GFP–streptavidin–NLS (GSN2). After fusion with polyethylene glycol, the cells were fixed and stained for myc-tagged proteins with 9E10 and a Texas red–conjugated secondary antibody. Nuclei were visualized using DAPI. The arrows denote the GSN2 cells in the fusion. Bar, 10 μm.
Figure 2.
Exportin-5 interacts with the RanGTPase and nucleoporins. (A) HF7c (MATa) yeast that express the indicated bait proteins as GAL4 DBD fusions were mated with the W303 (MATα) strain expressing the VP16 transactivation domain either alone (VP16) or as a fusion with Ran (VP16-Ran). Diploid yeast were selected on Leu−Trp− plates and replica plated onto Leu−Trp−His− ±3-aminotriazole (3AT) as indicated. (B) Exportin-5–His6 was incubated with either GST-RanGDP or GST-RanGTP bound to GSH beads. Bound proteins were separated by SDS-PAGE and detected by Coomassie staining. (C) The apparent dissociation constant of the RanGTP–exportin-5 complex was estimated using a RanGAP protection assay. Ran[γ-32P]GTP was preincubated for 15 min with the indicated concentrations of exportin-5–His6. The GTPase reaction was started by addition of RanGAP. After 3 min, the proteins were filter bound and washed. Hydrolysis of GTP was determined by the amount of Ran[γ-32P]GTP bound. Assays were in duplicate and are representative of two independent experiments. Error is expressed as the differences from the means. (D) HA–importin β and HA–exportin-5 were immunoprecipitated from HEK293 cell lysates to which RanQ69L was added as indicated. Untransfected HEK293 cells were used as a control for nonspecific nucleoporin binding (Mock IP). Lysate was prepared by solubilizing HEK293 cells with boiling sample buffer. Coimmunoprecipitated nucleoporins were detected with mAb RL1 (Snow et al., 1987). RL1 efficiently detected RanBP2, Can/Nup214, and Nup153 but not other nucleoporins in HEK293 lysate. HA–importin β and HA–exportin-5 were detected using mAb 12CA5. A protein not characterized previously as reacting with the RL1 mAb bound exportin-5 in the absence of RanQ69L and is denoted with an asterisk. (E) Heterotypic cell fusions were performed between BHK21 cells, transiently transfected with pKmyc–exportin-5, and a stably transfected HeLa cell line expressing GFP–streptavidin–NLS (GSN2). After fusion with polyethylene glycol, the cells were fixed and stained for myc-tagged proteins with 9E10 and a Texas red–conjugated secondary antibody. Nuclei were visualized using DAPI. The arrows denote the GSN2 cells in the fusion. Bar, 10 μm.
Figure 3.
Exportin-5 interacts with ILF3 in a RanGTP-dependent manner. (A) Schematic representation of human ILF3. Important elements found in this protein include the following: a putative bipartite NLS (striped box), two dsRBDs (shaded boxes), and an RGG RNA-binding motif (dotted box). The fragment that bound exportin-5 in the dihybrid screen is underlined. (B) Conjugation assays were performed as in the legend to Fig. 2 using VP16-ILF3(533–640) as the prey. Diploid yeast were replica plated onto selective medium (L−W−H−). (C) Equal amounts of GST or GST-ILF3(533–640) were immobilized on GSH beads and incubated with 200 nM recombinant exportin-5–His6 ±3 μM RanQ69L. An aliquot of the supernatant (1/10 the total) was analyzed to ensure the beads were incubated with equal concentrations of exportin-5–His6. Bound and unbound fractions were analyzed by SDS-PAGE and immunoblotting with anti-His6, anti-Ran, and anti-GST antibodies. All lanes were exposed equally to film. (D) HEK293 cells were transfected with full-length human pKH3-ILF3. Cells were lysed after 48 h. HA-tagged proteins were immunoprecipitated with 12CA5, immobilized on protein A–Sepharose, and then incubated with 200 nM exportin-5–His6 ±3 μM RanQ69L. HEK293 cells transfected with empty vector were used as a negative control. Bound proteins were analyzed by SDS-PAGE and immunoblotting with anti-His6 and anti-HA antibodies.
Figure 3.
Exportin-5 interacts with ILF3 in a RanGTP-dependent manner. (A) Schematic representation of human ILF3. Important elements found in this protein include the following: a putative bipartite NLS (striped box), two dsRBDs (shaded boxes), and an RGG RNA-binding motif (dotted box). The fragment that bound exportin-5 in the dihybrid screen is underlined. (B) Conjugation assays were performed as in the legend to Fig. 2 using VP16-ILF3(533–640) as the prey. Diploid yeast were replica plated onto selective medium (L−W−H−). (C) Equal amounts of GST or GST-ILF3(533–640) were immobilized on GSH beads and incubated with 200 nM recombinant exportin-5–His6 ±3 μM RanQ69L. An aliquot of the supernatant (1/10 the total) was analyzed to ensure the beads were incubated with equal concentrations of exportin-5–His6. Bound and unbound fractions were analyzed by SDS-PAGE and immunoblotting with anti-His6, anti-Ran, and anti-GST antibodies. All lanes were exposed equally to film. (D) HEK293 cells were transfected with full-length human pKH3-ILF3. Cells were lysed after 48 h. HA-tagged proteins were immunoprecipitated with 12CA5, immobilized on protein A–Sepharose, and then incubated with 200 nM exportin-5–His6 ±3 μM RanQ69L. HEK293 cells transfected with empty vector were used as a negative control. Bound proteins were analyzed by SDS-PAGE and immunoblotting with anti-His6 and anti-HA antibodies.
Figure 3.
Exportin-5 interacts with ILF3 in a RanGTP-dependent manner. (A) Schematic representation of human ILF3. Important elements found in this protein include the following: a putative bipartite NLS (striped box), two dsRBDs (shaded boxes), and an RGG RNA-binding motif (dotted box). The fragment that bound exportin-5 in the dihybrid screen is underlined. (B) Conjugation assays were performed as in the legend to Fig. 2 using VP16-ILF3(533–640) as the prey. Diploid yeast were replica plated onto selective medium (L−W−H−). (C) Equal amounts of GST or GST-ILF3(533–640) were immobilized on GSH beads and incubated with 200 nM recombinant exportin-5–His6 ±3 μM RanQ69L. An aliquot of the supernatant (1/10 the total) was analyzed to ensure the beads were incubated with equal concentrations of exportin-5–His6. Bound and unbound fractions were analyzed by SDS-PAGE and immunoblotting with anti-His6, anti-Ran, and anti-GST antibodies. All lanes were exposed equally to film. (D) HEK293 cells were transfected with full-length human pKH3-ILF3. Cells were lysed after 48 h. HA-tagged proteins were immunoprecipitated with 12CA5, immobilized on protein A–Sepharose, and then incubated with 200 nM exportin-5–His6 ±3 μM RanQ69L. HEK293 cells transfected with empty vector were used as a negative control. Bound proteins were analyzed by SDS-PAGE and immunoblotting with anti-His6 and anti-HA antibodies.
Figure 3.
Exportin-5 interacts with ILF3 in a RanGTP-dependent manner. (A) Schematic representation of human ILF3. Important elements found in this protein include the following: a putative bipartite NLS (striped box), two dsRBDs (shaded boxes), and an RGG RNA-binding motif (dotted box). The fragment that bound exportin-5 in the dihybrid screen is underlined. (B) Conjugation assays were performed as in the legend to Fig. 2 using VP16-ILF3(533–640) as the prey. Diploid yeast were replica plated onto selective medium (L−W−H−). (C) Equal amounts of GST or GST-ILF3(533–640) were immobilized on GSH beads and incubated with 200 nM recombinant exportin-5–His6 ±3 μM RanQ69L. An aliquot of the supernatant (1/10 the total) was analyzed to ensure the beads were incubated with equal concentrations of exportin-5–His6. Bound and unbound fractions were analyzed by SDS-PAGE and immunoblotting with anti-His6, anti-Ran, and anti-GST antibodies. All lanes were exposed equally to film. (D) HEK293 cells were transfected with full-length human pKH3-ILF3. Cells were lysed after 48 h. HA-tagged proteins were immunoprecipitated with 12CA5, immobilized on protein A–Sepharose, and then incubated with 200 nM exportin-5–His6 ±3 μM RanQ69L. HEK293 cells transfected with empty vector were used as a negative control. Bound proteins were analyzed by SDS-PAGE and immunoblotting with anti-His6 and anti-HA antibodies.
Figure 4.
Exportin-5 binds ILF3 specifically and in an LMB- insensitive manner. (A) Equal amounts of GST-ILF3(533–640) were immobilized on GSH beads and incubated with 200 nM exportin-5–His6, 500 nM Crm1-His6, or 200 nM importin β–His6 ±3 μM RanQ69L as indicated. Proteins were analyzed and detected as in the legend to Fig. 3. (B) Equal amounts of GST-PKI(36–50) or GST-ILF3(533–640) were immobilized on GSH beads. The beads were incubated with 200 nM Crm1-His6 or exportin-5–His6 ±3 μM RanQ69L. Where indicated, Crm1-His6 and exportin-5–His6 were incubated with 500 nM LMB at room temperature for 15 min before their addition to the binding assay. Proteins were analyzed and detected as described in the legend to Fig. 3. Exportin-5 is unable to bind GST-PKI(36–50) (unpublished data).
Figure 4.
Exportin-5 binds ILF3 specifically and in an LMB- insensitive manner. (A) Equal amounts of GST-ILF3(533–640) were immobilized on GSH beads and incubated with 200 nM exportin-5–His6, 500 nM Crm1-His6, or 200 nM importin β–His6 ±3 μM RanQ69L as indicated. Proteins were analyzed and detected as in the legend to Fig. 3. (B) Equal amounts of GST-PKI(36–50) or GST-ILF3(533–640) were immobilized on GSH beads. The beads were incubated with 200 nM Crm1-His6 or exportin-5–His6 ±3 μM RanQ69L. Where indicated, Crm1-His6 and exportin-5–His6 were incubated with 500 nM LMB at room temperature for 15 min before their addition to the binding assay. Proteins were analyzed and detected as described in the legend to Fig. 3. Exportin-5 is unable to bind GST-PKI(36–50) (unpublished data).
Figure 5.
Exportin-5 binds the dsRBDs of multiple proteins, but binding is blocked by dsRNA. (A) Equal amounts of GST-ILF3 dsRBD2, GST-Spnr dsRBD2, GST-staufen dsRBD3, or GST-PKR dsRBD1 were immobilized on GSH beads. Beads were incubated with 200 nM exportin-5–His6 ±3 μM RanQ69L. Proteins were analyzed and detected as described in the legend to Fig. 3. (B) Amino acid sequences of ILF3 dsRBD2, Spnr dsRBD2, staufen dsRBD3, and PKR dsRBD1 were aligned using CLUSTAL. Residues conserved in all four dsRBDs are in black. Closely related residues found in at least three of the four sequences are in gray. Regions of secondary structure are denoted as boxes above the sequences. Asterisks denote conserved residues in the three regions that make essential contacts with the RNA. Residues essential for the correct packing of the α-helices against the β sheet are marked with diamonds. (C) GST-ILF3(404–592) and GST-ILF3(404–592)mut (1 μg each) were separated by SDS-PAGE and stained using Coomassie blue. GST-ILF3(404–592)mut is a construct that is deficient in dsRNA binding. A duplicate gel was transferred to nitrocellulose. The immobilized proteins were incubated with an α-32P–labeled dsRNA probe. After washing to remove excess probe, the filter was exposed to film for ∼18 h before developing. (D) GST-ILF3(404–592) was immobilized on GSH-agarose beads and either ssRNA or dsRNA was added. After RNA binding, the beads were incubated with 100 nM exportin-5 ±3 μM RanQ69L. Proteins were analyzed and detected as described in the legend to Fig. 3.
Figure 5.
Exportin-5 binds the dsRBDs of multiple proteins, but binding is blocked by dsRNA. (A) Equal amounts of GST-ILF3 dsRBD2, GST-Spnr dsRBD2, GST-staufen dsRBD3, or GST-PKR dsRBD1 were immobilized on GSH beads. Beads were incubated with 200 nM exportin-5–His6 ±3 μM RanQ69L. Proteins were analyzed and detected as described in the legend to Fig. 3. (B) Amino acid sequences of ILF3 dsRBD2, Spnr dsRBD2, staufen dsRBD3, and PKR dsRBD1 were aligned using CLUSTAL. Residues conserved in all four dsRBDs are in black. Closely related residues found in at least three of the four sequences are in gray. Regions of secondary structure are denoted as boxes above the sequences. Asterisks denote conserved residues in the three regions that make essential contacts with the RNA. Residues essential for the correct packing of the α-helices against the β sheet are marked with diamonds. (C) GST-ILF3(404–592) and GST-ILF3(404–592)mut (1 μg each) were separated by SDS-PAGE and stained using Coomassie blue. GST-ILF3(404–592)mut is a construct that is deficient in dsRNA binding. A duplicate gel was transferred to nitrocellulose. The immobilized proteins were incubated with an α-32P–labeled dsRNA probe. After washing to remove excess probe, the filter was exposed to film for ∼18 h before developing. (D) GST-ILF3(404–592) was immobilized on GSH-agarose beads and either ssRNA or dsRNA was added. After RNA binding, the beads were incubated with 100 nM exportin-5 ±3 μM RanQ69L. Proteins were analyzed and detected as described in the legend to Fig. 3.
Figure 5.
Exportin-5 binds the dsRBDs of multiple proteins, but binding is blocked by dsRNA. (A) Equal amounts of GST-ILF3 dsRBD2, GST-Spnr dsRBD2, GST-staufen dsRBD3, or GST-PKR dsRBD1 were immobilized on GSH beads. Beads were incubated with 200 nM exportin-5–His6 ±3 μM RanQ69L. Proteins were analyzed and detected as described in the legend to Fig. 3. (B) Amino acid sequences of ILF3 dsRBD2, Spnr dsRBD2, staufen dsRBD3, and PKR dsRBD1 were aligned using CLUSTAL. Residues conserved in all four dsRBDs are in black. Closely related residues found in at least three of the four sequences are in gray. Regions of secondary structure are denoted as boxes above the sequences. Asterisks denote conserved residues in the three regions that make essential contacts with the RNA. Residues essential for the correct packing of the α-helices against the β sheet are marked with diamonds. (C) GST-ILF3(404–592) and GST-ILF3(404–592)mut (1 μg each) were separated by SDS-PAGE and stained using Coomassie blue. GST-ILF3(404–592)mut is a construct that is deficient in dsRNA binding. A duplicate gel was transferred to nitrocellulose. The immobilized proteins were incubated with an α-32P–labeled dsRNA probe. After washing to remove excess probe, the filter was exposed to film for ∼18 h before developing. (D) GST-ILF3(404–592) was immobilized on GSH-agarose beads and either ssRNA or dsRNA was added. After RNA binding, the beads were incubated with 100 nM exportin-5 ±3 μM RanQ69L. Proteins were analyzed and detected as described in the legend to Fig. 3.
Figure 5.
Exportin-5 binds the dsRBDs of multiple proteins, but binding is blocked by dsRNA. (A) Equal amounts of GST-ILF3 dsRBD2, GST-Spnr dsRBD2, GST-staufen dsRBD3, or GST-PKR dsRBD1 were immobilized on GSH beads. Beads were incubated with 200 nM exportin-5–His6 ±3 μM RanQ69L. Proteins were analyzed and detected as described in the legend to Fig. 3. (B) Amino acid sequences of ILF3 dsRBD2, Spnr dsRBD2, staufen dsRBD3, and PKR dsRBD1 were aligned using CLUSTAL. Residues conserved in all four dsRBDs are in black. Closely related residues found in at least three of the four sequences are in gray. Regions of secondary structure are denoted as boxes above the sequences. Asterisks denote conserved residues in the three regions that make essential contacts with the RNA. Residues essential for the correct packing of the α-helices against the β sheet are marked with diamonds. (C) GST-ILF3(404–592) and GST-ILF3(404–592)mut (1 μg each) were separated by SDS-PAGE and stained using Coomassie blue. GST-ILF3(404–592)mut is a construct that is deficient in dsRNA binding. A duplicate gel was transferred to nitrocellulose. The immobilized proteins were incubated with an α-32P–labeled dsRNA probe. After washing to remove excess probe, the filter was exposed to film for ∼18 h before developing. (D) GST-ILF3(404–592) was immobilized on GSH-agarose beads and either ssRNA or dsRNA was added. After RNA binding, the beads were incubated with 100 nM exportin-5 ±3 μM RanQ69L. Proteins were analyzed and detected as described in the legend to Fig. 3.
Figure 6.
Nuclear export of ILF3 is dependent on exportin-5 in intact cells. (A) Results of coupled reverse transcription and PCRs on BHK21 (B) and HeLa (H) cell RNA. All reactions contained 1 μg of RNA and either exportin-5 or glyceraldehyde-3-phosphate dehydrogenase gene-specific primers. A fraction of each reaction (1/20) was visualized on an ethidium bromide-stained agarose gel. GGIBF3(533–640) (18 μM) was injected into the nuclei of (B) HeLa cells or (C) BHK21 cells using fluorescent dextran (TRITC-dextran, 1 mg/ml) as an injection marker. Where indicated, GGIBF3 was coinjected with either 2 μM exportin-5–His6 or 2 μM Crm1-His6. All injected cells were incubated 30 min before fixation and visualization of the injected import substrate. Bars, 10 μm.
Figure 6.
Nuclear export of ILF3 is dependent on exportin-5 in intact cells. (A) Results of coupled reverse transcription and PCRs on BHK21 (B) and HeLa (H) cell RNA. All reactions contained 1 μg of RNA and either exportin-5 or glyceraldehyde-3-phosphate dehydrogenase gene-specific primers. A fraction of each reaction (1/20) was visualized on an ethidium bromide-stained agarose gel. GGIBF3(533–640) (18 μM) was injected into the nuclei of (B) HeLa cells or (C) BHK21 cells using fluorescent dextran (TRITC-dextran, 1 mg/ml) as an injection marker. Where indicated, GGIBF3 was coinjected with either 2 μM exportin-5–His6 or 2 μM Crm1-His6. All injected cells were incubated 30 min before fixation and visualization of the injected import substrate. Bars, 10 μm.
Figure 6.
Nuclear export of ILF3 is dependent on exportin-5 in intact cells. (A) Results of coupled reverse transcription and PCRs on BHK21 (B) and HeLa (H) cell RNA. All reactions contained 1 μg of RNA and either exportin-5 or glyceraldehyde-3-phosphate dehydrogenase gene-specific primers. A fraction of each reaction (1/20) was visualized on an ethidium bromide-stained agarose gel. GGIBF3(533–640) (18 μM) was injected into the nuclei of (B) HeLa cells or (C) BHK21 cells using fluorescent dextran (TRITC-dextran, 1 mg/ml) as an injection marker. Where indicated, GGIBF3 was coinjected with either 2 μM exportin-5–His6 or 2 μM Crm1-His6. All injected cells were incubated 30 min before fixation and visualization of the injected import substrate. Bars, 10 μm.
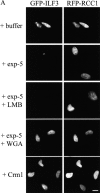
Figure 7.
Exportin-5 mediates export of ILF3 in digitonin- permeabilized cells. (A) HeLa cells were cotransfected with pEGFP-ILF3 and pKRed-RCC1. pKRed-RCC1 was used as a nuclear marker for transfected cells. The cells were permeabilized with digitonin, and export assays were performed in buffer alone or with buffer plus exportin-5–His6 (100 μg/ml), exportin-5–His6 (100 μg/ml) pretreated with LMB (1 μM), exportin-5–His6 (100 μg/ml) plus WGA (200 μg/ml), or Crm1-His6 (100 μg/ml). (B) Mean nuclear fluorescence values for GFP-ILF3 were obtained using Openlab (Improvision). All values were corrected for background fluorescence levels and normalized to the mean nuclear fluorescence levels of permeabilized buffer-treated cells. Each data point represents the mean nuclear fluorescence obtained from 30 randomly chosen cells. Error is expressed as ±1 SD from the mean. Bar, 10 μm.
Figure 7.
Exportin-5 mediates export of ILF3 in digitonin- permeabilized cells. (A) HeLa cells were cotransfected with pEGFP-ILF3 and pKRed-RCC1. pKRed-RCC1 was used as a nuclear marker for transfected cells. The cells were permeabilized with digitonin, and export assays were performed in buffer alone or with buffer plus exportin-5–His6 (100 μg/ml), exportin-5–His6 (100 μg/ml) pretreated with LMB (1 μM), exportin-5–His6 (100 μg/ml) plus WGA (200 μg/ml), or Crm1-His6 (100 μg/ml). (B) Mean nuclear fluorescence values for GFP-ILF3 were obtained using Openlab (Improvision). All values were corrected for background fluorescence levels and normalized to the mean nuclear fluorescence levels of permeabilized buffer-treated cells. Each data point represents the mean nuclear fluorescence obtained from 30 randomly chosen cells. Error is expressed as ±1 SD from the mean. Bar, 10 μm.
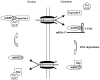
Figure 8.
Exportin-5 is a general export receptor for dsRBP. Exportin-5 and RanGTP associate with dsRBP in the nucleus. This export complex translocates through the NPC to the cytoplasm. RanGTP hydrolysis and binding to 3′ untranslated region elements on the target mRNA promotes release and retention of the dsRBP in the cytoplasm. RNA binding to the dsRBDs may inhibit import by preventing the interaction of an adjacent bipartite NLS with its nuclear import receptor (Imp). Release of the mRNA transcript due to RNA degradation would then permit import of the dsRBP. The shaded region of the dsRBP represents the dsRBD. A putative bipartite NLS is adjacent to the dsRBDs (see Fig. 3 A).
Similar articles
- Nucleocytoplasmic shuttling of JAZ, a new cargo protein for exportin-5.
Chen T, Brownawell AM, Macara IG. Chen T, et al. Mol Cell Biol. 2004 Aug;24(15):6608-19. doi: 10.1128/MCB.24.15.6608-6619.2004. Mol Cell Biol. 2004. PMID: 15254228 Free PMC article. - Minihelix-containing RNAs mediate exportin-5-dependent nuclear export of the double-stranded RNA-binding protein ILF3.
Gwizdek C, Ossareh-Nazari B, Brownawell AM, Evers S, Macara IG, Dargemont C. Gwizdek C, et al. J Biol Chem. 2004 Jan 9;279(2):884-91. doi: 10.1074/jbc.M306808200. Epub 2003 Oct 21. J Biol Chem. 2004. PMID: 14570900 - The karyopherin Kap142p/Msn5p mediates nuclear import and nuclear export of different cargo proteins.
Yoshida K, Blobel G. Yoshida K, et al. J Cell Biol. 2001 Feb 19;152(4):729-40. doi: 10.1083/jcb.152.4.729. J Cell Biol. 2001. PMID: 11266464 Free PMC article. - Distinct nuclear import and export pathways mediated by members of the karyopherin beta family.
Moroianu J. Moroianu J. J Cell Biochem. 1998 Aug 1;70(2):231-9. J Cell Biochem. 1998. PMID: 9671229 Review. - Functions of double-stranded RNA-binding domains in nucleocytoplasmic transport.
Banerjee S, Barraud P. Banerjee S, et al. RNA Biol. 2014;11(10):1226-32. doi: 10.4161/15476286.2014.972856. RNA Biol. 2014. PMID: 25584639 Free PMC article. Review.
Cited by
- Exploring Cellular Gateways: Unraveling the Secrets of Disordered Proteins within Live Nuclear Pores.
Yu W, Tingey M, Kelich JM, Li Y, Yu J, Junod SL, Jiang Z, Hansen I, Good N, Yang W. Yu W, et al. Res Sq [Preprint]. 2024 Jan 9:rs.3.rs-3504130. doi: 10.21203/rs.3.rs-3504130/v1. Res Sq. 2024. PMID: 38260360 Free PMC article. Preprint. - The competitive landscape of the dsRNA world.
Cottrell KA, Andrews RJ, Bass BL. Cottrell KA, et al. Mol Cell. 2024 Jan 4;84(1):107-119. doi: 10.1016/j.molcel.2023.11.033. Epub 2023 Dec 19. Mol Cell. 2024. PMID: 38118451 Review. - Lipid kinase PIP5K1A regulates let-7 microRNA biogenesis through interacting with nuclear export protein XPO5.
Li C, Yoon B, Stefani G, Slack FJ. Li C, et al. Nucleic Acids Res. 2023 Oct 13;51(18):9849-9862. doi: 10.1093/nar/gkad709. Nucleic Acids Res. 2023. PMID: 37655623 Free PMC article. - Regulated dicing of pre-mir-144 via reshaping of its terminal loop.
Shang R, Kretov DA, Adamson SI, Treiber T, Treiber N, Vedanayagam J, Chuang JH, Meister G, Cifuentes D, Lai EC. Shang R, et al. Nucleic Acids Res. 2022 Jul 22;50(13):7637-7654. doi: 10.1093/nar/gkac568. Nucleic Acids Res. 2022. PMID: 35801921 Free PMC article.
References
- Arts, G.J., M. Fornerod, and I.W. Mattaj. 1998. Identification of a nuclear export receptor for tRNA. Curr. Biol. 8:305–314. - PubMed
- Askjaer, P., T.H. Jensen, J. Nilsson, L. Englmeier, and J. Kjems. 1998. The specificity of the CRM1-Rev nuclear export signal interaction is mediated by RanGTP. J. Biol. Chem. 273:33414–33422. - PubMed
- Bashirullah, A., R.L. Cooperstock, and H.D. Lipshitz. 1998. RNA localization in development. Annu. Rev. Biochem. 67:335–394. - PubMed
- Bischoff, F.R., and H. Ponstingl. 1991. Catalysis of guanine nucleotide exchange on Ran by the mitotic regulator RCC1. Nature. 354:80–82. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous