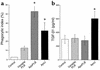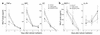Phosphatidylserine-dependent ingestion of apoptotic cells promotes TGF-beta1 secretion and the resolution of inflammation - PubMed (original) (raw)
Phosphatidylserine-dependent ingestion of apoptotic cells promotes TGF-beta1 secretion and the resolution of inflammation
Mai-Lan N Huynh et al. J Clin Invest. 2002 Jan.
Abstract
Ingestion of apoptotic cells in vitro by macrophages induces TGF-beta1 secretion, resulting in an anti-inflammatory effect and suppression of proinflammatory mediators. Here, we show in vivo that direct instillation of apoptotic cells enhanced the resolution of acute inflammation. This enhancement appeared to require phosphatidylserine (PS) on the apoptotic cells and local induction of TGF-beta1. Working with thioglycollate-stimulated peritonea or LPS-stimulated lungs, we examined the effect of apoptotic cell uptake on TGF-beta1 induction. Viable or opsonized apoptotic human Jurkat T cells, or apoptotic PLB-985 cells, human monomyelocytes that do not express PS during apoptosis, failed to induce TGF-beta1. PS liposomes, or PS directly transferred onto the PLB-985 surface membranes, restored the TGF-beta1 induction. Apoptotic cell instillation into LPS-stimulated lungs reduced proinflammatory chemokine levels in the bronchoalveolar lavage fluid (BALF). Additionally, total inflammatory cell counts in the BALF were markedly reduced 1-5 days after apoptotic cell instillation, an effect that could be reversed by opsonization or coinstillation of TGF-beta1 neutralizing antibody. This reduction resulted from early decrease in neutrophils and later decreases in lymphocytes and macrophages. In conclusion, apoptotic cell recognition and clearance, via exposure of PS and ligation of its receptor, induce TGF-beta1 secretion, resulting in accelerated resolution of inflammation.
Figures
Figure 1
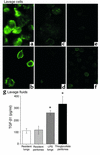
TGF-β1 detection in fresh lavage. (a–f) Freshly harvested cells were stained with chicken anti–hTGF-β1 IgY, followed by goat anti-chicken IgG Alexa 488. High levels of TGF-β1 were detected in (a) thioglycollate-elicited peritoneal (3 days old) and (b) LPS-elicited alveolar (2 days old) macrophages, but not in (c) resident peritoneal macrophages, (d) resident alveolar macrophages, or thioglycollate-elicited peritoneal macrophages (TPMφ’s) with (e) secondary antibody alone and (f) with isotype control. (g) Lavage supernatants from resident (unstimulated) lungs or peritonea had small amounts of TGF-β1, while LPS-stimulated lungs and thioglycollate-stimulated peritonea had high levels of TGF-β1 upon harvest. *P < 0.05, n ≥ 8, ± SD.
Figure 2
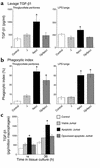
In vivo secretion of TGF-β1 in inflamed peritonea and lungs is increased by apoptotic cell clearance. After intraperitoneal or endotracheal instillation of HBSS alone (control), viable Jurkat T cells (J), apoptotic Jurkat T cells (ApoJ), or opsonized apoptotic Jurkat T cells (OpApoJ) into LPS-stimulated lungs (2 days old) and thioglycollate-stimulated peritonea (3 days old), lavage supernatants collected after 4 hours and 1 hour, respectively, of incubation were assayed for TGF-β1 by ELISA. (a) ApoJ induced TGF-β1 when compared with control, while J and OpApoJ did not. (b) Phagocytic index (PI = number of apoptotic bodies/200 macrophages × 100) of peritoneal or bronchoalveolar lavage fluid cytospins showed low uptake in the control and J, and increased PI for both ApoJ and OpApoJ. (c) The TPMφ’s were isolated and cultured after in vivo instillation of cells; induction of TGF-β1 in the macrophages treated with apoptotic Jurkat T cells persisted for 18 and 36 hours in tissue culture, when compared with that in viable and opsonized apoptotic Jurkat T cells. (a) *P < 0.05, n = 6, ± SD; (b) *P < 0.05, n ≥ 12, ± SD; (c) *P < 0.05, n ≥ 10, ± SD.
Figure 3
In vivo TGF-β1 induction resulted from increased release and de novo synthesis. (a) The increase in TGF-β1 secretion in thioglycollate-stimulated peritoneum was seen in 30-minute lavages after in vivo instillation of apoptotic Jurkat T cells (ApoJ) and was more pronounced than in the 4-hour lavages. (b) TPMφ cell-associated TGF-β1 at 1 hour was reduced after in vivo instillation of ApoJ. (c) Total (secreted + cell-associated) TGF-β1 remained constant. *P < 0.05, n = 8, ± SD. (d) In vitro secretion of TGF-β1 by TPMφ’s was increased after addition of apoptotic Jurkat T cells (ApoJ) compared with media alone (control) at 4 and 18 hours (*P < 0.05). TGF-β1 secretion in both control and ApoJ-treated macrophages was markedly inhibited by preincubation with cycloheximide (5 μg/ml) for 1 hour. **P < 0.001, n ≥ 13, ± SD.
Figure 4
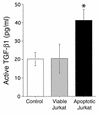
Active TGF-β1 in lavage fluids. Thioglycollate-stimulated peritonea were instilled with media alone (control), and viable or apoptotic Jurkat T cells. Lavage fluids obtained 1 hour later were assayed for TGF-β1 by ELISA without preactivation to determine active TGF-β1 levels. Apoptotic Jurkats significantly increased active TGF-β1 level compared with viable cells and control. *P < 0.05, n ≥ 12, ± SD.
Figure 5
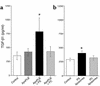
In vivo TGF-β1 induction by PLB-985 cells. Viable PLB or apoptotic PLB cells (ApoPLB) and apoptotic Jurkat T cells (ApoJ) were injected into thioglycollate-stimulated peritonea. In the lavage fluid and cells obtained 1 hour later, (a) phagocytic indexes were comparable in the ApoPLB and ApoJ groups. (b) Viable PLB and ApoPLB cells failed to induce TGF-β1, in comparison with ApoJ. *P < 0.02, n ≥ 10, ± SD.
Figure 6
In vivo TGF-β1 secretion in thioglycollate-stimulated peritoneum after instillation of apoptotic PLB-985 cells (ApoPLB) expressing phospholipid PS or PC in the outer leaflet of the plasma membrane. The peritonea were lavaged 1 hour after instillation of cells and TGF-β1 determined by ELISA. (a) ApoPLB that expressed PS (ApoPLB + PS) induced TGF-β1 when compared with HBSS (control), while unmodified ApoPLB (ApoPLB) or ApoPLB expressing PC (ApoPLB + PC) did not. *P < 0.05, n ≥ 9, ± SD. (b) In vivo TGF-β1 secretion in LPS-stimulated lungs after instillation of buffer (control), or PS or PC liposomes. TGF-β1 in BALF obtained 1 hour later was induced by PS liposomes above control levels, but not by PC liposomes. *P < 0.05, n ≥ 12, ± SD.
Figure 7
Cytokine and chemokine levels in BAL fluids from LPS-stimulated lungs detected by ELISA. One day after in vivo instillation of HBSS, viable Jurkat T cells, or apoptotic Jurkat T cells, apoptotic cells reduced TNF-α, MIP2, and KC levels (a), but not MCP-1 and IL-10 levels (b). *P < 0.05, n ≥ 25, ± SD. Levels continued to decline but no significant differences were found at days 3 and 5.
Figure 8
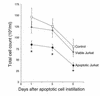
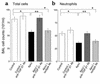
Reduction in BALF total cell counts. Total cell counts in BALF from LPS-stimulated lungs after in vivo instillation of HBSS, viable Jurkat T cells, or apoptotic Jurkat T cells. Apoptotic cells resulted in reduction of total cell counts at days 1, 3, and 5 compared with controls (buffer alone or viable cells). *P < 0.05, n ≥ 25, ± SD.
Figure 9
Reduction in BALF inflammatory cells. Cell differential in BALF from LPS-stimulated lungs 1, 3, and 5 days after in vivo instillation of HBSS (control) or viable or apoptotic Jurkat T cells. (a) Neutrophils were reduced at days 1–3, (b) lymphocytes were reduced at days 3 and 5, and (c) macrophages were decreased by day 5. *P < 0.05, **P < 0.07, n ≥ 25, ± SD.
Figure 10
Reduction in inflammation in the lungs. LPS-stimulated lung sections from 1 and 3 days after in vivo apoptotic Jurkat T cell instillation were fixed and stained with H&E. The most severely inflamed areas are shown for (a) day 1 after instillation of HBSS (control) and apoptotic Jurkat T cells (ApoJ), and (b) day 3 after instillation of HBSS (control) and apoptotic Jurkat T cells (ApoJ).
Figure 11
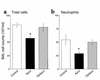
Opsonization reversed the anti-inflammatory effect of apoptotic cell clearance. Apoptotic Jurkat T cells were opsonized with mouse anti–human CD45 IgG prior to instillation into LPS-stimulated lungs. Opsonization completely reversed the reduction in BALF total cell (a) and neutrophil (b) counts seen one day after apoptotic cell clearance. *P < 0.05, n ≥ 15, ± SD.
Figure 12
Anti–TGF-β1 antibody abrogated the anti-inflammatory effect of apoptotic cell clearance. Goat anti–TGF-β1 neutralizing IgG (anti–TGF-β1 Ab) or normal goat IgG (Isotype Ab) were coinstilled with media alone (control) or apoptotic Jurkat T cells (ApoJ) into LPS-stimulated lungs. Day 1 BALF showed that the reduction in total cell counts (a) and neutrophils (b) by the ApoJ was completely reversed by opsonization and anti–TGF-β1 Ab but not isotype control. Coinstillation of anti–TGF-β1 Ab into the control group had no significant effect. *P < 0.05, **P < 0.05, n ≥ 20, ± SD.
Similar articles
- Vitamin E inhibits anti-Fas-induced phosphatidylserine oxidation but does not affect its externalization during apoptosis in Jurkat T cells and their phagocytosis by J774A.1 macrophages.
Serinkan BF, Tyurina YY, Babu H, Djukic M, Quinn PJ, Schroit A, Kagan VE. Serinkan BF, et al. Antioxid Redox Signal. 2004 Apr;6(2):227-36. doi: 10.1089/152308604322899297. Antioxid Redox Signal. 2004. PMID: 15025924 - The central role of phosphatidylserine in the phagocytosis of apoptotic thymocytes.
Schlegel RA, Callahan MK, Williamson P. Schlegel RA, et al. Ann N Y Acad Sci. 2000;926:217-25. doi: 10.1111/j.1749-6632.2000.tb05614.x. Ann N Y Acad Sci. 2000. PMID: 11193037 Review. - An Apoptotic 'Eat Me' Signal: Phosphatidylserine Exposure.
Segawa K, Nagata S. Segawa K, et al. Trends Cell Biol. 2015 Nov;25(11):639-650. doi: 10.1016/j.tcb.2015.08.003. Epub 2015 Oct 1. Trends Cell Biol. 2015. PMID: 26437594 Review.
Cited by
- Identification of Axl as a downstream effector of TGF-β1 during Langerhans cell differentiation and epidermal homeostasis.
Bauer T, Zagórska A, Jurkin J, Yasmin N, Köffel R, Richter S, Gesslbauer B, Lemke G, Strobl H. Bauer T, et al. J Exp Med. 2012 Oct 22;209(11):2033-47. doi: 10.1084/jem.20120493. Epub 2012 Oct 15. J Exp Med. 2012. PMID: 23071254 Free PMC article. - Amelioration of Nicotine-Induced Osteoarthritis by Platelet-Derived Biomaterials Through Modulating IGF-1/AKT/IRS-1 Signaling Axis.
Lo WC, Dubey NK, Tsai FC, Lu JH, Peng BY, Chiang PC, Singh AK, Wu CY, Cheng HC, Deng WP. Lo WC, et al. Cell Transplant. 2020 Jan-Dec;29:963689720947348. doi: 10.1177/0963689720947348. Cell Transplant. 2020. PMID: 32757664 Free PMC article. - Resolvin D1 Programs Inflammation Resolution by Increasing TGF-β Expression Induced by Dying Cell Clearance in Experimental Autoimmune Neuritis.
Luo B, Han F, Xu K, Wang J, Liu Z, Shen Z, Li J, Liu Y, Jiang M, Zhang ZY, Zhang Z. Luo B, et al. J Neurosci. 2016 Sep 14;36(37):9590-603. doi: 10.1523/JNEUROSCI.0020-16.2016. J Neurosci. 2016. PMID: 27629711 Free PMC article. - Annexin-V promotes anti-tumor immunity and inhibits neuroblastoma growth in vivo.
Yan X, Doffek K, Yin C, Krein M, Phillips M, Sugg SL, Johnson B, Shilyansky J. Yan X, et al. Cancer Immunol Immunother. 2012 Nov;61(11):1917-27. doi: 10.1007/s00262-012-1250-4. Epub 2012 Apr 5. Cancer Immunol Immunother. 2012. PMID: 22476407 Free PMC article. - SIGN-R1, a C-type lectin, enhances apoptotic cell clearance through the complement deposition pathway by interacting with C1q in the spleen.
Prabagar MG, Do Y, Ryu S, Park JY, Choi HJ, Choi WS, Yun TJ, Moon J, Choi IS, Ko K, Ko K, Young Shin C, Cheong C, Kang YS. Prabagar MG, et al. Cell Death Differ. 2013 Apr;20(4):535-45. doi: 10.1038/cdd.2012.160. Epub 2012 Dec 14. Cell Death Differ. 2013. PMID: 23238564 Free PMC article.
References
- Fadok VA, et al. Exposure of phosphatidylserine on the surface of apoptotic lymphocytes triggers specific recognition and removal by macrophages. J Immunol. 1992; 148:2207–2216. - PubMed
- Frasch SC, et al. Regulation of phospholipid scramblase activity during apoptosis and cell activation by protein kinase Cdelta. J Biol Chem. 2000; 275:23065–23073. - PubMed
- Bratton DL, et al. Appearance of phosphatidylserine on apoptotic cells requires calcium-mediated nonspecific flip-flop and is enhanced by loss of the aminophospholipid translocase. J Biol Chem. 1997; 272:26159–26165. - PubMed
- Fadok VA, et al. A receptor for phosphatidylserine-specific clearance of apoptotic cells. Nature. 2000; 405:85–90. - PubMed
- Savill J. Apoptosis in resolution of inflammation. Kidney Blood Press Res. 2000; 23:173–174. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources