Association of BAFF/BLyS overexpression and altered B cell differentiation with Sjögren's syndrome - PubMed (original) (raw)
Comparative Study
doi: 10.1172/JCI14121.
Susan L Kalled, Anne H Cutler, Carl Olson, Stephen A Woodcock, Pascal Schneider, Jurg Tschopp, Teresa G Cachero, Marcel Batten, Julie Wheway, Davide Mauri, Dana Cavill, Tom P Gordon, Charles R Mackay, Fabienne Mackay
Affiliations
- PMID: 11781351
- PMCID: PMC150825
- DOI: 10.1172/JCI14121
Comparative Study
Association of BAFF/BLyS overexpression and altered B cell differentiation with Sjögren's syndrome
Joanna Groom et al. J Clin Invest. 2002 Jan.
Abstract
BAFF (BLyS, TALL-1, THANK, zTNF4) is a member of the TNF superfamily that specifically regulates B lymphocyte proliferation and survival. Mice transgenic (Tg) for BAFF develop an autoimmune condition similar to systemic lupus erythematosus. We now demonstrate that BAFF Tg mice, as they age, develop a secondary pathology reminiscent of Sjögren's syndrome (SS), which is manifested by severe sialadenitis, decreased saliva production, and destruction of submaxillary glands. In humans, SS also correlates with elevated levels of circulating BAFF, as well as a dramatic upregulation of BAFF expression in inflamed salivary glands. A likely explanation for disease in BAFF Tg mice is excessive survival signals to autoreactive B cells, possibly as they pass through a critical tolerance checkpoint while maturing in the spleen. The marginal zone (MZ) B cell compartment, one of the enlarged B cell subsets in the spleen of BAFF Tg mice, is a potential reservoir of autoreactive B cells. Interestingly, B cells with an MZ-like phenotype infiltrate the salivary glands of BAFF Tg mice, suggesting that cells of this compartment potentially participate in tissue damage in SS and possibly other autoimmune diseases. We conclude that altered B cell differentiation and tolerance induced by excess BAFF may be central to SS pathogenesis.
Figures
Figure 1
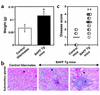
Enlarged and inflamed salivary glands in BAFF Tg mice. (a) Mice 15–17 months old (four control littermates and four BAFF Tg mice) were sacrificed the same day for organ collection. Both right and left submaxillary glands were collected and weighed. The data shows the combined weight (mean ± SD) of both glands. These results are representative of at least four separate groups of dissected age-matched animals. (b) Paraffin sections of submaxillary glands from a control littermate (left panel) and two BAFF Tg mice (middle and right panels) were stained with hematoxylin and eosin. Arrows indicate ducts and acinar cells in the left and right panels. The arrow in the middle panel shows acinar destruction. Asterisks indicate periductal infiltrates (foci). Magnification: ×100. (c) Paraffin sections of submaxillary glands from seven control mice and 22 BAFF Tg mice (12–17 months old) were prepared as shown in b and scored for disease as described in Methods. Bars indicate the mean disease score for each group. *P < 0.05, **P < 0.03.
Figure 2
Identification of MZ-like B cells infiltrating salivary glands of BAFF Tg mice. Lymphocytes were isolated from salivary glands of BAFF Tg mice (13–17 months old) and control littermates. Cells were stained with anti-B220, anti-CD5, anti-IgM, and anti-CD43 using multi-color flow cytometry. (a) Gates used to identify B220hi and B220lo/int B cells in control and BAFF Tg mice. (b) Expression of IgM and CD5 on B220hi cells and B220lo/int cells from BAFF Tg and control mice. The IgMhi B cell gate on B220hi cells is boxed and the B-1a and B-1b gates on B220lo/int cells are indicated. (c) Expression of CD43 on gated B220hi/IgMhi cells and gated B-1a and B-1b cells from a BAFF Tg mouse. (d) L-selectin expression on lymphocytes from salivary gland and inguinal lymph node of a BAFF Tg mouse, gated on B220hi/IgMhi cells and on IgM+ cells. (e) Lymphocytes from a BAFF Tg mouse and a control littermate were prepared as in a and stained with anti-B220, anti-IgM, and antibodies to the indicated markers. Cells were gated on B220hi and IgMhi cells, as shown in the top histograms. In c–e, mean fluorescence intensity (MFI) and bars delineating negative control staining are indicated. (f) Schematic representation of the common and distinct markers expressed on MZ B cells (right circle), B-1 cells (left circle), and T1 B cells (lower circle). The phenotype of the B cells infiltrating submaxillary glands of BAFF Tg mice is represented by the dotted ellipse. These results are representative of 12 BAFF Tg mice and seven control mice analyzed. FSC, forward light scatter.
Figure 3
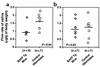
Decreased saliva flow in older BAFF Tg mice. Thirteen BAFF Tg mice (diamonds) and 14 control littermates (circles) were injected with pilocarpine prior to saliva collection as described in Methods. (a) Mice 13–15.5 months old. (b) Mice 8–10 months old. Mean values for saliva flow are shown with a bar. P values are indicated in each panel.
Figure 4
Elevated levels of BAFF in sera and salivary gland tissues from patients suffering from primary SS; no correlation with levels of total IgG, RF, and presence of anti-Ro/La autoantibodies. (a) Individual serum BAFF levels in 39 healthy controls (squares), 41 patients with primary SS (diamonds), 53 patients with SLE (circles), and 53 patients with RA (triangles) were measured by ELISA. The horizontal black bars indicate the mean for each group: normal 10.4 ± 13 (ng/ml); SS 53 ± 67 (ng/ml); SLE 12.7 ± 24.4 (ng/ml); and RA 23 ± 47 (ng/ml). Sera of 16 normal individuals, 6 SS, 20 SLE, and 10 RA patients had no detectable levels of BAFF. The dotted line delineates the range of normal BAFF levels. *P < 0.04, as determined by ANOVA. (b and c) Correlation of serum BAFF levels in patients with SS with the levels of IgG (b) and RF (c) in each serum sample. R and P values were calculated by ANOVA. (d) Levels of BAFF in patients with anti-Ro plus anti-La or anti-Ro only, and in patients with no precipitin detected (Neg). (e) Paraffin sections of a human labial gland biopsy from a patient with SS were stained with anti-human BAFF antibody or an isotype-matched control antibody. Magnification: ×200. Staining of normal human labial gland with anti-human BAFF antibody is also shown (magnification: ×100). These pictures are representative of four patients with primary SS and three control tissues analyzed. RF, rheumatoid factors.
Comment in
- The buzz about BAFF.
Vaux DL. Vaux DL. J Clin Invest. 2002 Jan;109(1):17-8. doi: 10.1172/JCI14780. J Clin Invest. 2002. PMID: 11781346 Free PMC article. No abstract available.
Similar articles
- Development of nephritis but not sialadenitis in autoimmune-prone BAFF transgenic mice lacking marginal zone B cells.
Fletcher CA, Sutherland AP, Groom JR, Batten ML, Ng LG, Gommerman J, Mackay F. Fletcher CA, et al. Eur J Immunol. 2006 Sep;36(9):2504-14. doi: 10.1002/eji.200636270. Eur J Immunol. 2006. PMID: 16906535 - B-cell tolerance breakdown in Sjögren's syndrome: focus on BAFF.
Varin MM, Le Pottier L, Youinou P, Saulep D, Mackay F, Pers JO. Varin MM, et al. Autoimmun Rev. 2010 Jul;9(9):604-8. doi: 10.1016/j.autrev.2010.05.006. Epub 2010 May 8. Autoimmun Rev. 2010. PMID: 20457281 Review. - The BAFF/APRIL system: an important player in systemic rheumatic diseases.
Mackay F, Sierro F, Grey ST, Gordon TP. Mackay F, et al. Curr Dir Autoimmun. 2005;8:243-65. doi: 10.1159/000082106. Curr Dir Autoimmun. 2005. PMID: 15564724 Review. - Expression of BAFF (BLyS) in T cells infiltrating labial salivary glands from patients with Sjögren's syndrome.
Lavie F, Miceli-Richard C, Quillard J, Roux S, Leclerc P, Mariette X. Lavie F, et al. J Pathol. 2004 Apr;202(4):496-502. doi: 10.1002/path.1533. J Pathol. 2004. PMID: 15095277 - Development of autoimmune nephritis in genetically asplenic and splenectomized BAFF transgenic mice.
Fletcher CA, Groom JR, Woehl B, Leung H, Mackay C, Mackay F. Fletcher CA, et al. J Autoimmun. 2011 Mar;36(2):125-34. doi: 10.1016/j.jaut.2010.12.002. Epub 2011 Jan 7. J Autoimmun. 2011. PMID: 21216131
Cited by
- The role of cytokines from salivary gland epithelial cells in the immunopathology of Sjögren's syndrome.
Dong Y, Wang T, Wu H. Dong Y, et al. Front Immunol. 2024 Sep 13;15:1443455. doi: 10.3389/fimmu.2024.1443455. eCollection 2024. Front Immunol. 2024. PMID: 39346911 Free PMC article. Review. - Efficacy of BAFF inhibition and B-cell depletion in non-obese diabetic mice as a spontaneous model for Sjögren's disease.
Felten R, Foray AP, Schneider P, Marquet C, Pecquet C, Monneaux F, Dumortier H, Sibilia J, Valette F, Chatenoud L, Gottenberg JE. Felten R, et al. RMD Open. 2024 Aug 28;10(3):e004112. doi: 10.1136/rmdopen-2024-004112. RMD Open. 2024. PMID: 39209370 Free PMC article. - Splenic marginal zone B cells restrict Mycobacterium tuberculosis infection by shaping the cytokine pattern and cell-mediated immunity.
Tsai CY, Oo M, Peh JH, Yeo BCM, Aptekmann A, Lee B, Liu JJJ, Tsao WS, Dick T, Fink K, Gengenbacher M. Tsai CY, et al. Cell Rep. 2024 Jul 23;43(7):114426. doi: 10.1016/j.celrep.2024.114426. Epub 2024 Jul 2. Cell Rep. 2024. PMID: 38959109 Free PMC article. - Investigative biological therapies for primary Sjögren's syndrome.
He SH, Xuan YY, Liang X, Guo KJ, Wang Y, Fu XN, Li TF. He SH, et al. Arch Med Sci. 2020 Jul 15;20(2):506-516. doi: 10.5114/aoms.2020.97288. eCollection 2024. Arch Med Sci. 2020. PMID: 38757038 Free PMC article. - Iguratimod suppresses plasma cell differentiation and ameliorates experimental Sjögren's syndrome in mice by promoting TEC kinase degradation.
Yang YQ, Liu YJ, Qiao WX, Jin W, Zhu SW, Yan YX, Luo Q, Xu Q. Yang YQ, et al. Acta Pharmacol Sin. 2024 Sep;45(9):1926-1936. doi: 10.1038/s41401-024-01288-7. Epub 2024 May 14. Acta Pharmacol Sin. 2024. PMID: 38744938
References
- Jonsson, R., Haga, H.-J., and Gordon, T.P. 2000. Sjögren’s syndrome. In Arthritis and allied conditions. W. Koopman, editor. Lippincott Williams & Wilkins. Philadelphia, Pennsylvania, USA. 1736–1759.
- Manoussakis, M.N., Talal, N., and Moutsopoulos, H.M. 1998. Sjögren’s syndrome. In The autoimmune diseases. N.R. Rose and I.R. Mackay, editors. Academic Press. San Diego, California, USA. 381–404.
- Haneji N, et al. Identification of α-fodrin as a candidate autoantigen in primary Sjögren’s syndrome. Science. 1997; 276:604–607. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Miscellaneous