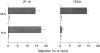Rapid acquisition of tissue-specific homing phenotypes by CD4(+) T cells activated in cutaneous or mucosal lymphoid tissues - PubMed (original) (raw)
Rapid acquisition of tissue-specific homing phenotypes by CD4(+) T cells activated in cutaneous or mucosal lymphoid tissues
Daniel J Campbell et al. J Exp Med. 2002.
Abstract
Effector and memory T cells can be subdivided based on their ability to traffic through peripheral tissues such as inflamed skin and intestinal lamina propria, a property controlled by expression of 'tissue-specific' adhesion and chemoattractant receptors. However, little is known about the development of these selectively homing T cell subsets, and it is unclear whether activation in cutaneous versus intestinal lymphoid organs directly results in effector/memory T cells that differentially express adhesion and chemoattractant receptors targeting them to the corresponding nonlymphoid site. We define two murine CD4(+) effector/memory T cell subsets that preferentially localize in cutaneous or intestinal lymphoid organs by their reciprocal expression of the adhesion molecules P-selectin ligand (P-lig) and alpha 4 beta 7, respectively. We show that within 2 d of systemic immunization CD4(+) T cells activated in cutaneous lymph nodes upregulate P-lig, and downregulate alpha 4 beta 7, while those responding to antigen in intestinal lymph nodes selectively express high levels of alpha 4 beta 7 and acquire responsiveness to the intestinal chemokine thymus-expressed chemokine (TECK). Thus, during an immune response, local microenvironments within cutaneous and intestinal secondary lymphoid organs differentially direct T cell expression of these adhesion and chemoattractant receptors, targeting the resulting effector T cells to the inflamed skin or intestinal lamina propria.
Figures
Figure 1.
α4β7 and P-lig define three CD4+ memory T cell populations that differentially localize in intestinal and cutaneous lymphoid organs. (Left) CD4 and CD45RB expression by lymphocytes isolated from the cutaneous PLNs of a >1-y-old BALB/c mouse. The gates used to define ‘naive’ (CD4+CD45RB+) and ‘memory’ (CD4+CD45RB−) T cells are shown. (Right) α4β7 and P-lig expression by gated naive and memory CD4+ T cells isolated from the indicated lymphoid tissues of a >1-y-old BALB/c mouse.
Figure 2.
P-lig expression does not distinguish Th1, Th2, and Th0 cells analyzed directly ex vivo. (A, top) IFN-γ and IL-4 expression by gated CD4+ T cells determined by intracellular cytokine staining and flow cytometry after 4 h of PMA plus ionomycin stimulation of lymphocytes isolated from the indicated organs. (Bottom) Staining of stimulated lymphocytes from PLNs with isotype control antibodies. (B) Percentage of P-lig+ cells among gated Th1, Th2, and Th0 CD4+ T cells isolated from the indicated organs. Each data point represents a measurement taken from an individual animal. A total of three >1-y-old BALB/c mice were analyzed.
Figure 2.
P-lig expression does not distinguish Th1, Th2, and Th0 cells analyzed directly ex vivo. (A, top) IFN-γ and IL-4 expression by gated CD4+ T cells determined by intracellular cytokine staining and flow cytometry after 4 h of PMA plus ionomycin stimulation of lymphocytes isolated from the indicated organs. (Bottom) Staining of stimulated lymphocytes from PLNs with isotype control antibodies. (B) Percentage of P-lig+ cells among gated Th1, Th2, and Th0 CD4+ T cells isolated from the indicated organs. Each data point represents a measurement taken from an individual animal. A total of three >1-y-old BALB/c mice were analyzed.
Figure 3.
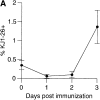
CD4+ T cells activated in PLNs and MLNs differentially upregulate P-lig and α4β7. (A) Percentage of OVA-specific KJ1–26+ cells among gated CD4+ T cells isolated from peripheral blood at the indicated times after intraperitoneal injection of OVA plus LPS. Data are mean and SD of values obtained from five mice at each time point. (B) Representative flow cytometry data of cellular CFSE content and KJ1–26 staining on gated CD4+ T cells isolated from the indicated tissues 2 d after intraperitoneal immunization of DO11.10 adoptive transfer recipients with OVA plus LPS. The horizontal marker in the histograms represents the CFSE fluorescence intensity of naive CD4+KJ1–26+ T cells isolated from animals immunized with LPS alone (data not shown). (C) Expression of α4β7 and P-lig by gated CD4+KJ1–26+ cells isolated from PLNs (• and ○) and MLNs (▪ and □) 2 d after intraperitoneal immunization of DO11.10 adoptive transfer recipients with OVA plus LPS (black symbols) or LPS alone (white symbols). Each data point represents a measurement from an individual animal. (D) Representative flow cytometry data of α4β7 and P-lig staining on gated CD4+KJ1–26+ cells isolated from PLNs (top) and MLN (bottom) 2 d after immunization of DO11.10 adoptive transfer recipients with OVA plus LPS (left) or LPS alone (right). The quadrant gate used to define the P-lig+ and α4β7hi populations in C is indicated. (E) Mean fluorescence intensity (MFI) of α4β7 (left) and P-lig (right) staining on gated CD4+KJ1–26+ cells isolated from the MLNs (▪) or PLNs (•) 2 d after intraperitoneal immunization of DO11.10 adoptive transfer recipients with OVA plus LPS as a function of cell division (as determined by CFSE content). Data are mean and SE of values obtained from four (α4β7) or five (P-lig) mice. N represents the MFI of α4β7 or P-lig staining on naive cells isolated from animals immunized with LPS alone. Dotted lines represent background MFI of cells stained with an isotype control (left) or unstained cells (right).
Figure 3.
CD4+ T cells activated in PLNs and MLNs differentially upregulate P-lig and α4β7. (A) Percentage of OVA-specific KJ1–26+ cells among gated CD4+ T cells isolated from peripheral blood at the indicated times after intraperitoneal injection of OVA plus LPS. Data are mean and SD of values obtained from five mice at each time point. (B) Representative flow cytometry data of cellular CFSE content and KJ1–26 staining on gated CD4+ T cells isolated from the indicated tissues 2 d after intraperitoneal immunization of DO11.10 adoptive transfer recipients with OVA plus LPS. The horizontal marker in the histograms represents the CFSE fluorescence intensity of naive CD4+KJ1–26+ T cells isolated from animals immunized with LPS alone (data not shown). (C) Expression of α4β7 and P-lig by gated CD4+KJ1–26+ cells isolated from PLNs (• and ○) and MLNs (▪ and □) 2 d after intraperitoneal immunization of DO11.10 adoptive transfer recipients with OVA plus LPS (black symbols) or LPS alone (white symbols). Each data point represents a measurement from an individual animal. (D) Representative flow cytometry data of α4β7 and P-lig staining on gated CD4+KJ1–26+ cells isolated from PLNs (top) and MLN (bottom) 2 d after immunization of DO11.10 adoptive transfer recipients with OVA plus LPS (left) or LPS alone (right). The quadrant gate used to define the P-lig+ and α4β7hi populations in C is indicated. (E) Mean fluorescence intensity (MFI) of α4β7 (left) and P-lig (right) staining on gated CD4+KJ1–26+ cells isolated from the MLNs (▪) or PLNs (•) 2 d after intraperitoneal immunization of DO11.10 adoptive transfer recipients with OVA plus LPS as a function of cell division (as determined by CFSE content). Data are mean and SE of values obtained from four (α4β7) or five (P-lig) mice. N represents the MFI of α4β7 or P-lig staining on naive cells isolated from animals immunized with LPS alone. Dotted lines represent background MFI of cells stained with an isotype control (left) or unstained cells (right).
Figure 3.
CD4+ T cells activated in PLNs and MLNs differentially upregulate P-lig and α4β7. (A) Percentage of OVA-specific KJ1–26+ cells among gated CD4+ T cells isolated from peripheral blood at the indicated times after intraperitoneal injection of OVA plus LPS. Data are mean and SD of values obtained from five mice at each time point. (B) Representative flow cytometry data of cellular CFSE content and KJ1–26 staining on gated CD4+ T cells isolated from the indicated tissues 2 d after intraperitoneal immunization of DO11.10 adoptive transfer recipients with OVA plus LPS. The horizontal marker in the histograms represents the CFSE fluorescence intensity of naive CD4+KJ1–26+ T cells isolated from animals immunized with LPS alone (data not shown). (C) Expression of α4β7 and P-lig by gated CD4+KJ1–26+ cells isolated from PLNs (• and ○) and MLNs (▪ and □) 2 d after intraperitoneal immunization of DO11.10 adoptive transfer recipients with OVA plus LPS (black symbols) or LPS alone (white symbols). Each data point represents a measurement from an individual animal. (D) Representative flow cytometry data of α4β7 and P-lig staining on gated CD4+KJ1–26+ cells isolated from PLNs (top) and MLN (bottom) 2 d after immunization of DO11.10 adoptive transfer recipients with OVA plus LPS (left) or LPS alone (right). The quadrant gate used to define the P-lig+ and α4β7hi populations in C is indicated. (E) Mean fluorescence intensity (MFI) of α4β7 (left) and P-lig (right) staining on gated CD4+KJ1–26+ cells isolated from the MLNs (▪) or PLNs (•) 2 d after intraperitoneal immunization of DO11.10 adoptive transfer recipients with OVA plus LPS as a function of cell division (as determined by CFSE content). Data are mean and SE of values obtained from four (α4β7) or five (P-lig) mice. N represents the MFI of α4β7 or P-lig staining on naive cells isolated from animals immunized with LPS alone. Dotted lines represent background MFI of cells stained with an isotype control (left) or unstained cells (right).
Figure 3.
CD4+ T cells activated in PLNs and MLNs differentially upregulate P-lig and α4β7. (A) Percentage of OVA-specific KJ1–26+ cells among gated CD4+ T cells isolated from peripheral blood at the indicated times after intraperitoneal injection of OVA plus LPS. Data are mean and SD of values obtained from five mice at each time point. (B) Representative flow cytometry data of cellular CFSE content and KJ1–26 staining on gated CD4+ T cells isolated from the indicated tissues 2 d after intraperitoneal immunization of DO11.10 adoptive transfer recipients with OVA plus LPS. The horizontal marker in the histograms represents the CFSE fluorescence intensity of naive CD4+KJ1–26+ T cells isolated from animals immunized with LPS alone (data not shown). (C) Expression of α4β7 and P-lig by gated CD4+KJ1–26+ cells isolated from PLNs (• and ○) and MLNs (▪ and □) 2 d after intraperitoneal immunization of DO11.10 adoptive transfer recipients with OVA plus LPS (black symbols) or LPS alone (white symbols). Each data point represents a measurement from an individual animal. (D) Representative flow cytometry data of α4β7 and P-lig staining on gated CD4+KJ1–26+ cells isolated from PLNs (top) and MLN (bottom) 2 d after immunization of DO11.10 adoptive transfer recipients with OVA plus LPS (left) or LPS alone (right). The quadrant gate used to define the P-lig+ and α4β7hi populations in C is indicated. (E) Mean fluorescence intensity (MFI) of α4β7 (left) and P-lig (right) staining on gated CD4+KJ1–26+ cells isolated from the MLNs (▪) or PLNs (•) 2 d after intraperitoneal immunization of DO11.10 adoptive transfer recipients with OVA plus LPS as a function of cell division (as determined by CFSE content). Data are mean and SE of values obtained from four (α4β7) or five (P-lig) mice. N represents the MFI of α4β7 or P-lig staining on naive cells isolated from animals immunized with LPS alone. Dotted lines represent background MFI of cells stained with an isotype control (left) or unstained cells (right).
Figure 3.
CD4+ T cells activated in PLNs and MLNs differentially upregulate P-lig and α4β7. (A) Percentage of OVA-specific KJ1–26+ cells among gated CD4+ T cells isolated from peripheral blood at the indicated times after intraperitoneal injection of OVA plus LPS. Data are mean and SD of values obtained from five mice at each time point. (B) Representative flow cytometry data of cellular CFSE content and KJ1–26 staining on gated CD4+ T cells isolated from the indicated tissues 2 d after intraperitoneal immunization of DO11.10 adoptive transfer recipients with OVA plus LPS. The horizontal marker in the histograms represents the CFSE fluorescence intensity of naive CD4+KJ1–26+ T cells isolated from animals immunized with LPS alone (data not shown). (C) Expression of α4β7 and P-lig by gated CD4+KJ1–26+ cells isolated from PLNs (• and ○) and MLNs (▪ and □) 2 d after intraperitoneal immunization of DO11.10 adoptive transfer recipients with OVA plus LPS (black symbols) or LPS alone (white symbols). Each data point represents a measurement from an individual animal. (D) Representative flow cytometry data of α4β7 and P-lig staining on gated CD4+KJ1–26+ cells isolated from PLNs (top) and MLN (bottom) 2 d after immunization of DO11.10 adoptive transfer recipients with OVA plus LPS (left) or LPS alone (right). The quadrant gate used to define the P-lig+ and α4β7hi populations in C is indicated. (E) Mean fluorescence intensity (MFI) of α4β7 (left) and P-lig (right) staining on gated CD4+KJ1–26+ cells isolated from the MLNs (▪) or PLNs (•) 2 d after intraperitoneal immunization of DO11.10 adoptive transfer recipients with OVA plus LPS as a function of cell division (as determined by CFSE content). Data are mean and SE of values obtained from four (α4β7) or five (P-lig) mice. N represents the MFI of α4β7 or P-lig staining on naive cells isolated from animals immunized with LPS alone. Dotted lines represent background MFI of cells stained with an isotype control (left) or unstained cells (right).
Figure 4.
CD4+ T cells activated in PLNs or MLNs differentially respond to the intestinal chemokine TECK. Migration of CD4+KJ1–26+ cells isolated from the MLNs or PLNs 2 d after intraperitoneal immunization of DO11.10 adoptive transfer recipients with OVA plus LPS to medium alone (white bars) or to the indicated chemokines (gray bars). Data are mean and SD of multiple measurements taken in one experiment, which is representative of 4.
Similar articles
- CCR7 expression and memory T cell diversity in humans.
Campbell JJ, Murphy KE, Kunkel EJ, Brightling CE, Soler D, Shen Z, Boisvert J, Greenberg HB, Vierra MA, Goodman SB, Genovese MC, Wardlaw AJ, Butcher EC, Wu L. Campbell JJ, et al. J Immunol. 2001 Jan 15;166(2):877-84. doi: 10.4049/jimmunol.166.2.877. J Immunol. 2001. PMID: 11145663 - Gut-associated lymphoid tissue-primed CD4+ T cells display CCR9-dependent and -independent homing to the small intestine.
Stenstad H, Ericsson A, Johansson-Lindbom B, Svensson M, Marsal J, Mack M, Picarella D, Soler D, Marquez G, Briskin M, Agace WW. Stenstad H, et al. Blood. 2006 May 1;107(9):3447-54. doi: 10.1182/blood-2005-07-2860. Epub 2006 Jan 3. Blood. 2006. PMID: 16391017 - Differential expression of lymphocyte homing receptors by human memory/effector T cells in pulmonary versus cutaneous immune effector sites.
Picker LJ, Martin RJ, Trumble A, Newman LS, Collins PA, Bergstresser PR, Leung DY. Picker LJ, et al. Eur J Immunol. 1994 Jun;24(6):1269-77. doi: 10.1002/eji.1830240605. Eur J Immunol. 1994. PMID: 7515808 - Generation of gut-homing T cells and their localization to the small intestinal mucosa.
Johansson-Lindbom B, Agace WW. Johansson-Lindbom B, et al. Immunol Rev. 2007 Feb;215:226-42. doi: 10.1111/j.1600-065X.2006.00482.x. Immunol Rev. 2007. PMID: 17291292 Review. - Chemokine-mediated control of T cell traffic in lymphoid and peripheral tissues.
Ebert LM, Schaerli P, Moser B. Ebert LM, et al. Mol Immunol. 2005 May;42(7):799-809. doi: 10.1016/j.molimm.2004.06.040. Epub 2004 Nov 23. Mol Immunol. 2005. PMID: 15829268 Review.
Cited by
- Protective immunity against Chlamydia trachomatis can engage both CD4+ and CD8+ T cells and bridge the respiratory and genital mucosae.
Nogueira CV, Zhang X, Giovannone N, Sennott EL, Starnbach MN. Nogueira CV, et al. J Immunol. 2015 Mar 1;194(5):2319-29. doi: 10.4049/jimmunol.1402675. Epub 2015 Jan 30. J Immunol. 2015. PMID: 25637024 Free PMC article. - A molecular mucosal adjuvant to enhance immunity against pneumococcal infection in the elderly.
Fukuyama Y, Ikeda Y, Ohori J, Sugita G, Aso K, Fujihashi K, Briles DE, McGhee JR, Fujihashi K. Fukuyama Y, et al. Immune Netw. 2015 Feb;15(1):9-15. doi: 10.4110/in.2015.15.1.9. Epub 2015 Feb 17. Immune Netw. 2015. PMID: 25713504 Free PMC article. Review. - Oral Administration of Cancer Vaccines: Challenges and Future Perspectives.
Gambirasi M, Safa A, Vruzhaj I, Giacomin A, Sartor F, Toffoli G. Gambirasi M, et al. Vaccines (Basel). 2023 Dec 26;12(1):26. doi: 10.3390/vaccines12010026. Vaccines (Basel). 2023. PMID: 38250839 Free PMC article. Review. - Persistence and function of central and effector memory CD4+ T cells following infection with a gastrointestinal helminth.
Zaph C, Rook KA, Goldschmidt M, Mohrs M, Scott P, Artis D. Zaph C, et al. J Immunol. 2006 Jul 1;177(1):511-8. doi: 10.4049/jimmunol.177.1.511. J Immunol. 2006. PMID: 16785548 Free PMC article. - Human cerebrospinal fluid contains CD4+ memory T cells expressing gut- or skin-specific trafficking determinants: relevance for immunotherapy.
Kivisäkk P, Tucky B, Wei T, Campbell JJ, Ransohoff RM. Kivisäkk P, et al. BMC Immunol. 2006 Jul 7;7:14. doi: 10.1186/1471-2172-7-14. BMC Immunol. 2006. PMID: 16824229 Free PMC article.
References
- Butcher, E.C., and L.J. Picker. 1996. Lymphocyte homing and homeostasis. Science. 272:60–66. - PubMed
- Butcher, E.C., M. Williams, K. Youngman, L. Rott, and M. Briskin. 1999. Lymphocyte trafficking and regional immunity. Adv. Immunol. 72:209–253. - PubMed
- Rott, L.S., M.J. Briskin, D.P. Andrew, E.L. Berg, and E.C. Butcher. 1996. A fundamental subdivision of circulating lymphocytes defined by adhesion to mucosal addressin cell adhesion molecule-1. Comparison with vascular cell adhesion molecule-1 and correlation with β7 integrins and memory differentiation. J. Immunol. 156:3727–3736. - PubMed
- Bargatze, R.F., M.A. Jutila, and E.C. Butcher. 1995. Distinct roles of L-selectin and integrins α4β7 and LFA-1 in lymphocyte homing to Peyer's patch-HEV in situ: the multistep model confirmed and refined. Immunity. 3:99–108. - PubMed
- Zabel, B.A., W.W. Agace, J.J. Campbell, H.M. Heath, D. Parent, A.I. Roberts, E.C. Ebert, N. Kassam, S. Qin, M. Zovko, et al. 1999. Human G protein-coupled receptor GPR-9-6/CC chemokine receptor 9 is selectively expressed on intestinal homing T lymphocytes, mucosal lymphocytes, and thymocytes and is required for thymus-expressed chemokine-mediated chemotaxis. J. Exp. Med. 190:1241–1256. - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials