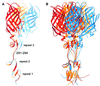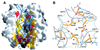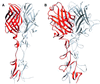Crystal structure of reovirus attachment protein sigma1 reveals evolutionary relationship to adenovirus fiber - PubMed (original) (raw)
Comparative Study
Crystal structure of reovirus attachment protein sigma1 reveals evolutionary relationship to adenovirus fiber
James D Chappell et al. EMBO J. 2002.
Abstract
Reovirus attaches to cellular receptors with the sigma1 protein, a fiber-like molecule protruding from the 12 vertices of the icosahedral virion. The crystal structure of a receptor-binding fragment of sigma1 reveals an elongated trimer with two domains: a compact head with a new beta-barrel fold and a fibrous tail containing a triple beta-spiral. Numerous structural and functional similarities between reovirus sigma1 and the adenovirus fiber suggest an evolutionary link in the receptor-binding strategies of these two viruses. A prominent loop in the sigma1 head contains a cluster of residues that are conserved among reovirus serotypes and are likely to form a binding site for junction adhesion molecule, an integral tight junction protein that serves as a reovirus receptor. The fibrous tail is mainly responsible for sigma1 trimer formation, and it contains a highly flexible region that allows for significant movement between the base of the tail and the head. The architecture of the trimer interface and the observed flexibility indicate that sigma1 is a metastable structure poised to undergo conformational changes upon viral attachment and cell entry.
Figures
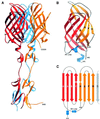
Fig. 1. Structure of reovirus σ1. (A) Ribbon drawing of the σ1 trimer. The three σ1 monomers are shown in red, orange and blue. Each monomer consists of a head domain formed by a compact β-barrel and a fibrous tail with three β-spiral repeats. Note the kink between the 3-fold axes of the head and tail domains. (B) Enlarged view of the σ1 head domain. The two Greek key motifs, shown in red and orange, form a compact, cylindrical β-sheet that contains eight β-strands (A–H). With the exception of the DE loop, the connections between the β-strands are very tight. The head domain also contains two short helices (blue): one 310 and one α-helix. (C) Schematic view of the β-strand arrangement in the σ1 head domain. Colors are as in (B); an additional D strand is shown in gray to depict the circular nature of the barrel. Strand D forms β-sheet-type hydrogen bonds with strands A and G.
Fig. 2. Flexibility of σ1. (A) Superposition of the three σ1 monomers present in the crystals. The superposition is based on the N-terminal two β-spiral repeats and reveals flexibility in the linker region (residues 291–294) between repeats 2 and 3. (B) Superposition, as in (A), using σ1 trimers. This representation highlights the degree of flexibility within the σ1 trimer as it protrudes from the virion. Three σ1 trimers are shown in red, orange and blue.
Fig. 3. Conservation of σ1 residues and predicted interaction with JAM. (A) Sequence alignment of T1L, T2J and T3D σ1. Conserved residues predicted to interact with JAM are shown in red; other conserved residues are shown in orange. (B) Surface representation of two σ1 monomers, with the third monomer shown as a blue ribbon. The conserved residues from (A) were mapped onto the σ1 surface using the same color code. The three views differ by rotations of 90° and 180°, respectively, along a vertical axis.
Fig. 4. The σ1 head trimer interface. (A) View into the head trimer interface. Two monomers are shown as surface representations, and the third monomer is shown as a blue ribbon. Surface residues that are within 4 Å of residues in the third monomer are shown in red (residues conserved in T1L, T2J and T3D σ1) and yellow (residues unique to T3D σ1). The contact area involving conserved residues Val344, Asp345 and Asp346 is boxed, and this region is shown in more detail in (B). (B) View along the trimer axis, centered at conserved residues Asp345 and Asp346 (yellow) located at the base of the head. Residues Tyr313, Arg314 and Tyr347 engage in contacts with the two aspartic acids. The side chains of Asp345 are likely to be protonated to avoid an accumulation of negative charge at the interface. Hydrogen bonds involving protonated Asp345 are indicated. Oxygen and nitrogen atoms of side chains are shown as red and blue spheres, respectively, and the Asp346 main chain amides are shown as blue spheres as well.
Fig. 5. Comparison of reovirus σ1 and adenovirus fiber structures. Trimeric structures of reovirus σ1 (A) and adenovirus fiber (B; van Raaij et al., 1999). In each case, one of the monomers is shown in red. Both attachment proteins have head-and-tail morphology, with a triple β-spiral forming the tail. The spirals of the crystallized reovirus σ1 fragment and adenovirus fiber (van Raaij et al., 1999) contain three and four repeats, respectively. (C) Superposition, in stereo, of σ1 head (red) and adenovirus fiber knob (blue; van Raaij et al., 1999). A portion of the β-spiral is shown in each case. The spirals emerge from the head domains in similar orientations and directions. In addition to the conserved β-sheet topology, the head and knob structures share a short helix (indicated in orange for σ1) in a long loop at the domain base.
Fig. 5. Comparison of reovirus σ1 and adenovirus fiber structures. Trimeric structures of reovirus σ1 (A) and adenovirus fiber (B; van Raaij et al., 1999). In each case, one of the monomers is shown in red. Both attachment proteins have head-and-tail morphology, with a triple β-spiral forming the tail. The spirals of the crystallized reovirus σ1 fragment and adenovirus fiber (van Raaij et al., 1999) contain three and four repeats, respectively. (C) Superposition, in stereo, of σ1 head (red) and adenovirus fiber knob (blue; van Raaij et al., 1999). A portion of the β-spiral is shown in each case. The spirals emerge from the head domains in similar orientations and directions. In addition to the conserved β-sheet topology, the head and knob structures share a short helix (indicated in orange for σ1) in a long loop at the domain base.
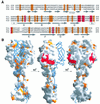
Fig. 6. Structure of the β-spiral repeats in the reovirus σ1 tail. (A) Detailed view of a section of the triple β-spiral of σ1. One σ1 chain is shown in orange; the other two are in gray. Residues Leu270, Met272, Ile274, Leu279 and Ile281 (blue) are located in β-strands and participate in the formation of a hydrophobic core that stabilizes the spiral. Residues Ser277 (blue) and Thr278 (magenta) are located in a β-turn that connects the two β-strands. The position of Thr278 is usually occupied by a proline or a glycine residue in β-spirals (van Raaij et al., 1999). (B) Sequence alignment of a region of the σ1 tail, indicating that the tail contains at least eight β-spiral repeats. The hydrophobic residues characteristic of β-spirals are indicated in blue, and the residues (usually proline or glycine) in the β-turns are shown in magenta. The consensus sequence for β-spiral repeats is shown at the bottom (h, hydrophobic residue; G, glycine; P, proline). Residues Asn198, Arg202 and Pro204 (shown in red) have been implicated in the interaction of T3D σ1 with sialic acid (Chappell et al., 1997). Arg245 (shown in blue) is the cleavage site for trypsin (Chappell et al., 1998). (C) Model of the complete spiral of σ1. Based on the sequence analysis shown in (B), the β-spiral probably begins at residue 167 of T3D σ1 and comprises eight repeats. The N-terminal five repeats are shown in gray. These repeats are not included in the crystal structure; the spiral has been extended using translated and rotated σ1 repeats to generate a model that depicts the approximate dimensions of the molecule. Residues prior to 167 are not shown; these residues are predicted to form an α-helical coiled-coil structure (Bassel-Duby et al., 1985; Duncan et al., 1990; Fraser et al., 1990; Nibert et al., 1990).
Similar articles
- Structural and Functional Features of the Reovirus σ1 Tail.
Dietrich MH, Ogden KM, Long JM, Ebenhoch R, Thor A, Dermody TS, Stehle T. Dietrich MH, et al. J Virol. 2018 Jun 29;92(14):e00336-18. doi: 10.1128/JVI.00336-18. Print 2018 Jul 15. J Virol. 2018. PMID: 29695426 Free PMC article. - Structural similarities in the cellular receptors used by adenovirus and reovirus.
Stehle T, Dermody TS. Stehle T, et al. Viral Immunol. 2004;17(2):129-43. doi: 10.1089/0882824041310621. Viral Immunol. 2004. PMID: 15279694 Review. - Structural evidence for common functions and ancestry of the reovirus and adenovirus attachment proteins.
Stehle T, Dermody TS. Stehle T, et al. Rev Med Virol. 2003 Mar-Apr;13(2):123-32. doi: 10.1002/rmv.379. Rev Med Virol. 2003. PMID: 12627395 Free PMC article. Review. - An Unusual Aspartic Acid Cluster in the Reovirus Attachment Fiber σ1 Mediates Stability at Low pH and Preserves Trimeric Organization.
Glorani G, Ruwolt M, Holton N, Loll B, Neu U. Glorani G, et al. J Virol. 2022 Apr 27;96(8):e0033122. doi: 10.1128/jvi.00331-22. Epub 2022 Apr 5. J Virol. 2022. PMID: 35380459 Free PMC article. - Junctional adhesion molecule a serves as a receptor for prototype and field-isolate strains of mammalian reovirus.
Campbell JA, Schelling P, Wetzel JD, Johnson EM, Forrest JC, Wilson GA, Aurrand-Lions M, Imhof BA, Stehle T, Dermody TS. Campbell JA, et al. J Virol. 2005 Jul;79(13):7967-78. doi: 10.1128/JVI.79.13.7967-7978.2005. J Virol. 2005. PMID: 15956543 Free PMC article.
Cited by
- Structure and assembly of complex viruses.
San Martín C. San Martín C. Subcell Biochem. 2013;68:329-60. doi: 10.1007/978-94-007-6552-8_11. Subcell Biochem. 2013. PMID: 23737057 Free PMC article. Review. - Adenoviruses as vaccine vectors.
Tatsis N, Ertl HC. Tatsis N, et al. Mol Ther. 2004 Oct;10(4):616-29. doi: 10.1016/j.ymthe.2004.07.013. Mol Ther. 2004. PMID: 15451446 Free PMC article. Review. - Structure and N-acetylglucosamine binding of the distal domain of mouse adenovirus 2 fibre.
Singh AK, Nguyen TH, Vidovszky MZ, Harrach B, Benkő M, Kirwan A, Joshi L, Kilcoyne M, Berbis MÁ, Cañada FJ, Jiménez-Barbero J, Menéndez M, Wilson SS, Bromme BA, Smith JG, van Raaij MJ. Singh AK, et al. J Gen Virol. 2018 Nov;99(11):1494-1508. doi: 10.1099/jgv.0.001145. Epub 2018 Oct 2. J Gen Virol. 2018. PMID: 30277856 Free PMC article. - Molecular Basis of Bacterial Host Interactions by Gram-Positive Targeting Bacteriophages.
Dunne M, Hupfeld M, Klumpp J, Loessner MJ. Dunne M, et al. Viruses. 2018 Jul 28;10(8):397. doi: 10.3390/v10080397. Viruses. 2018. PMID: 30060549 Free PMC article. Review. - Sequence analysis of the genome of piscine orthoreovirus (PRV) associated with heart and skeletal muscle inflammation (HSMI) in Atlantic salmon (Salmo salar).
Markussen T, Dahle MK, Tengs T, Løvoll M, Finstad ØW, Wiik-Nielsen CR, Grove S, Lauksund S, Robertsen B, Rimstad E. Markussen T, et al. PLoS One. 2013 Jul 29;8(7):e70075. doi: 10.1371/journal.pone.0070075. Print 2013. PLoS One. 2013. PMID: 23922911 Free PMC article.
References
- Barton E.S., Connolly,L., Forrest,J.C., Chappell,J.D. and Dermody,T.S. (2001a) Utilization of sialic acid as a co-receptor enhances reovirus attachment by multistep adhesion strengthening. J. Biol. Chem., 276, 2200–2211. - PubMed
- Barton E.S., Forrest,J.C., Connolly,J.L., Chappell,J.D., Liu,Y., Schnell,F.J., Nusrat,A., Parkos,C.A. and Dermody,T.S. (2001b) Junction adhesion molecule is a receptor for reovirus. Cell, 104, 441–451. - PubMed
- Bassel-Duby R., Jayasuriya,A., Chatterjee,D., Sonenberg,N., Maizel,J.V.,Jr and Fields,B.N. (1985) Sequence of reovirus haemagglutinin predicts a coiled-coil structure. Nature, 315, 421–423. - PubMed
- Benson S.D., Bamford,J.K., Bamford,D.H. and Burnett,R.M. (1999) Viral evolution revealed by bacteriophage PRD1 and human adenovirus coat protein structures. Cell, 98, 825–833. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- AI38296/AI/NIAID NIH HHS/United States
- R01 AI045716/AI/NIAID NIH HHS/United States
- R01 AI038296/AI/NIAID NIH HHS/United States
- P30 DK020593/DK/NIDDK NIH HHS/United States
- P30 CA068485/CA/NCI NIH HHS/United States
- CA68485/CA/NCI NIH HHS/United States
- R37 AI038296/AI/NIAID NIH HHS/United States
- DK20593/DK/NIDDK NIH HHS/United States
- AI45716/AI/NIAID NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources