Four plasmepsins are active in the Plasmodium falciparum food vacuole, including a protease with an active-site histidine - PubMed (original) (raw)
Four plasmepsins are active in the Plasmodium falciparum food vacuole, including a protease with an active-site histidine
Ritu Banerjee et al. Proc Natl Acad Sci U S A. 2002.
Abstract
Hemoglobin degradation is a metabolic process that is central to the growth and maturation of the malaria parasite Plasmodium falciparum. Two aspartic proteases that initiate degradation, plasmepsins (PMs) I and II, have been identified and extensively characterized. Eight additional PM genes are present in the P. falciparum genome. To better understand the enzymology of hemoglobin degradation, it is necessary to determine which of these genes are expressed when hemoglobin degradation is occurring, which encode active enzymes, and which gene products are found in the food vacuole where catabolism takes place. Our genome-wide analysis reveals that PM I, II, and IV and histo-aspartic protease encode hemoglobin-degrading food vacuole proteases. Despite having a histidine in place of one of the catalytic aspartic acids conserved in other aspartic proteases, histo-aspartic protease is an active hydrolase.
Figures
Figure 1
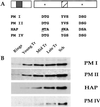
PM localization in intraerythrocytic stages. (A) Indirect immunofluorescence microscopy analysis of PMs. Phase/contrast (PC) and fluorescence (FL) images of trophozoites stained with antibodies to PM I, II, IV, V, IX, and X and HAP are shown. The dark regions in the phase/contrast images are hemozoin crystals within the FV. (B) Immunoelectron microscopy of trophozoites using anti-PM I, II, and IV and HAP antibodies. All four panels are shown at the same magnification. (Scale bar = 0.25 μm.) (Inset) Close-up of membranes surrounding the FV; arrowhead, RBC membrane; black arrow, parasitophorous vacuolar membrane; gray arrow, parasite plasma membrane; white arrow, FV membrane. The dark crystals within the FV are hemozoin.
Figure 2
PM I, II, and IV and HAP are similar in sequence and expression pattern. (A) Schematic diagram of PM I, II, and IV and HAP features. Each protein contains pro and mature regions. A transmembrane domain (shaded box) is present in the prosegment. Two active-site motifs (*) and the loop, known as the flap (hatched box), are shown in the mature proteins. Active-site amino acid substitutions in HAP are evident, most notably D32H and Y75S (underlined and italics). (B) Stage-specific expression of PMs. Blots containing equal numbers of ring, young, mid or late trophozoite (Tr), and schizont (Sch) stage parasites were probed with antibodies to PM I, II, and IV and HAP.
Figure 3
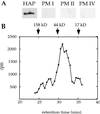
Characterization of purified HAP. (A) Active fractions from a 3G2–5 HAP immunoaffinity column contain only HAP. Eluant was trichloroacetic acid-precipitated, resolved by SDS/PAGE, blotted, and probed separately with antibodies to HAP and PM I, II, and IV. (B) Purified HAP migrates as a monomer of 35 kDa on gel filtration chromatography. HAP purified by DEAE and Mono S chromatography was injected onto a Superdex-200 column. Fractions were assayed for ability to cleave [14C]globin. The migration of molecular weight standards are indicated.
Figure 4
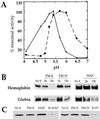
Properties of HAP and PM IV. (A) pH sensitivity of HAP and PM IV activity. Cleavage of α33–34 by each enzyme was monitored in 100 mM citrate-phosphate buffers ranging from pH 3.5 to 6.6. At pH 7.0, 100 mM [bis(2-hydroxyethyl)amino]tris(hydroxymethyl)methane was used. A saturating substrate concentration (2.5 μM) was used. Dashed line, HAP; solid line, PM IV. (B) Hemoglobin and globin cleavage by HAP and PM IV. Human hemoglobin or acid-denatured globin were incubated without enzyme (No E) or with 50 nM recombinant PM II, recombinant PM IV, or native HAP for 2 or 12 h in 100 mM citrate-phosphate, pH 5.0 (PM II), pH 5.4 (PM IV), or pH 5.7 (HAP). The amount of substrate remaining was determined by silver staining. (C) A combination of enzymes degrades hemoglobin efficiently. Human hemoglobin was incubated at 37°C for 2 h without enzyme (No E) or with 50 nM recombinant PM II, recombinant PM IV, native HAP, or a combination of enzymes. Assays were performed in 100 mM citrate-phosphate, pH 5.7 (HAP and PM II) or pH 5.4 (PM IV and PM II). Gels were developed with silver staining.
Similar articles
- Kinetic analysis of plasmepsins I and II aspartic proteases of the Plasmodium falciparum digestive vacuole.
Luker KE, Francis SE, Gluzman IY, Goldberg DE. Luker KE, et al. Mol Biochem Parasitol. 1996 Jul;79(1):71-8. doi: 10.1016/0166-6851(96)02651-5. Mol Biochem Parasitol. 1996. PMID: 8844673 - Biosynthesis and maturation of the malaria aspartic hemoglobinases plasmepsins I and II.
Francis SE, Banerjee R, Goldberg DE. Francis SE, et al. J Biol Chem. 1997 Jun 6;272(23):14961-8. doi: 10.1074/jbc.272.23.14961. J Biol Chem. 1997. PMID: 9169469 - Four aspartic proteases occur in the Plasmodium falciparum food vacuole.
Egan TJ. Egan TJ. Trends Parasitol. 2002 Apr;18(4):150. doi: 10.1016/s1471-4922(02)02265-1. Trends Parasitol. 2002. PMID: 11998697 No abstract available. - Structural studies of vacuolar plasmepsins.
Bhaumik P, Gustchina A, Wlodawer A. Bhaumik P, et al. Biochim Biophys Acta. 2012 Jan;1824(1):207-23. doi: 10.1016/j.bbapap.2011.04.008. Epub 2011 Apr 20. Biochim Biophys Acta. 2012. PMID: 21540129 Free PMC article. Review. - Studies on plasmepsins I and II from the malarial parasite Plasmodium falciparum and their exploitation as drug targets.
Moon RP, Bur D, Loetscher H, D'Arcy A, Tyas L, Oefner C, Grueninger-Leitch F, Mona D, Rupp K, Dorn A, Matile H, Certa U, Berry C, Kay J, Ridley RG. Moon RP, et al. Adv Exp Med Biol. 1998;436:397-406. doi: 10.1007/978-1-4615-5373-1_56. Adv Exp Med Biol. 1998. PMID: 9561248 Review. No abstract available.
Cited by
- The Glucose Transporter PfHT1 Is an Antimalarial Target of the HIV Protease Inhibitor Lopinavir.
Kraft TE, Armstrong C, Heitmeier MR, Odom AR, Hruz PW. Kraft TE, et al. Antimicrob Agents Chemother. 2015 Oct;59(10):6203-9. doi: 10.1128/AAC.00899-15. Epub 2015 Jul 27. Antimicrob Agents Chemother. 2015. PMID: 26248369 Free PMC article. - Critical role of amino acid 23 in mediating activity and specificity of vinckepain-2, a papain-family cysteine protease of rodent malaria parasites.
Singh A, Shenai BR, Choe Y, Gut J, Sijwali PS, Craik CS, Rosenthal PJ. Singh A, et al. Biochem J. 2002 Nov 15;368(Pt 1):273-81. doi: 10.1042/BJ20020753. Biochem J. 2002. PMID: 12169096 Free PMC article. - Tackling resistance: emerging antimalarials and new parasite targets in the era of elimination.
Mathews ES, Odom John AR. Mathews ES, et al. F1000Res. 2018 Aug 1;7:F1000 Faculty Rev-1170. doi: 10.12688/f1000research.14874.1. eCollection 2018. F1000Res. 2018. PMID: 30135714 Free PMC article. Review. - PTEX helps efficiently traffic haemoglobinases to the food vacuole in Plasmodium falciparum.
Jonsdottir TK, Elsworth B, Cobbold S, Gabriela M, Ploeger E, Parkyn Schneider M, Charnaud SC, Dans MG, McConville M, Bullen HE, Crabb BS, Gilson PR. Jonsdottir TK, et al. PLoS Pathog. 2023 Jul 31;19(7):e1011006. doi: 10.1371/journal.ppat.1011006. eCollection 2023 Jul. PLoS Pathog. 2023. PMID: 37523385 Free PMC article. - Enzymatic and structural characterization of HAD5, an essential phosphomannomutase of malaria-causing parasites.
Frasse PM, Miller JJ, Polino AJ, Soleimani E, Zhu JS, Jakeman DL, Jez JM, Goldberg DE, Odom John AR. Frasse PM, et al. J Biol Chem. 2022 Feb;298(2):101550. doi: 10.1016/j.jbc.2021.101550. Epub 2021 Dec 29. J Biol Chem. 2022. PMID: 34973333 Free PMC article.
References
- Trigg P I, Kondrachine A V. Malaria: Parasite Biology, Pathogenesis and Protection. Washington, DC: Am. Soc. Microbiol.; 1998.
- Rosenthal P J. Adv Parasitol. 1999;43:105–159. - PubMed
- Banerjee R, Goldberg D E. In: Antimalarial Chemotherapy: Mechanisms of Action, Resistance, and New Directions in Drug Discovery. Rosenthal P J, editor. Totowa, NJ: Humana; 2000. pp. 43–63.
- Goldberg D E. Semin Cell Biol. 1993;4:355–361. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases