Multipotent neural stem cells reside into the rostral extension and olfactory bulb of adult rodents - PubMed (original) (raw)
Multipotent neural stem cells reside into the rostral extension and olfactory bulb of adult rodents
Angela Gritti et al. J Neurosci. 2002.
Abstract
The lateral walls of the forebrain lateral ventricles are the richest source of stem cells in the adult mammalian brain. These stem cells give rise to new olfactory neurons that are renewed throughout life. The neurons originate in the subventricular zone (SVZ), migrate within the rostral extension (RE) of the SVZ along the rostral migratory stream (RMS) within tube-like structures formed of glial cells, to eventually reach the olfactory bulb (OB). We demonstrate that, contrary to the current view, multipotential (neuronal-astroglial-oligodendroglial) precursors with stem cell features can be isolated not only from the SVZ but also from the entire RE, including the distal portion within the OB. Specifically, these stem cells do not derive from the migratory neuroblasts coming from the SVZ. Interestingly, stem cells isolated from the proximal RE generate significantly more oligodendrocytes, and those from the distal RE proliferate significantly more slowly than stem cells derived from the SVZ and other RE regions. These findings demonstrate that stem cells are not confined to the forebrain periventricular region and indicate that stem cells endowed with different functional characteristics occur at different levels of the SVZ-RE pathway.
Figures
Fig. 1.
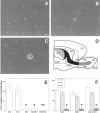
Undifferentiated cells isolated from the RE of adult mice proliferate in response to GFs. The forebrain of adult mice was dissected out, and three different regions were isolated (D): SVZ, subventricular zone;RE1, rostral extension in olfactory peduncle;RE2, rostral extension in the OB. SVZ–RE tissue is indicated in black (the dotted area shows the ventricular wall); RE tissue of the OB obtained by microdissection (not including the surrounding parenchyma) is indicated in_gray_. LV, Lateral ventricle;CX, cortex; CC, corpus callosum;PAR, parenchyma. Cells were cultured in the presence of EGF, FGF-2, or both; the number of spheres formed in each well was counted after 7–12 DIV. A, Hypertrophic cell from RE2 after 4 DIV proliferates and gives rise after 8 DIV to a small cluster of proliferating cells (B). After 12 DIV a primary sphere is formed (C). _E,_Spheres were generated from cells isolated from all three regions, but significantly fewer were obtained from RE2-derived cultures compared with those derived from SVZ and RE1. Microdissection and separation of the RE2 region into RE tissue (RE2/RE) and surrounding parenchyma (RE2/PAR), followed by culturing, showed that only cells dissociated from RE2/RE gave rise to spheres.F, Use of EGF, FGF-2, or both generated closely similar numbers of spheres in each of the three regions. Data are means ± SD of four independent experiments in triplicate. Tissue from two mice was pooled in each experiment. Scale bar, 20 μm. *Significantly different from SVZ and RE1; **significantly different from RE2/RE; Student's t test, p < 0.05.
Fig. 2.
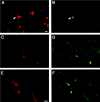
Cells isolated from the RE of adult mice are multipotent. Primary RE-generated spheres were dissociated, single cells were transferred to individual wells by micromanipulation (1 cell per well) in growth medium, and followed by time-lapse microphotography. Virtually all of the cells that were classified as single cells by visual inspection in our clonal assays were indeed single cells, as confirmed by the detection of a single nucleus by DAPI staining (A, inset). The cell shown in A(2 hr after plating; derived from an RE2-primary sphere) proliferated (B; 7 d) and gave rise after 20 d to a spherical clone of cells (primary clonal sphere; C). Primary clonal spheres were subcloned to generate secondary clonal spheres, which were pooled and serially passaged to generate a clonal cell line. After differentiation by removal of GFs, the progeny of RE-derived clonal cell lines included neuronal, astroglial, and oligodendroglial cells. D, E, Phase-contrast and fluorescence micrographs of differentiated cultures from the RE2-derived clonal cell line A7.16. Triple-labeling immunofluorescence revealed the simultaneous presence of neurons (Tuj1,green; filled arrowheads), astrocytes (GFAP, blue; arrows), and oligodendrocytes (O4, red; open arrowheads) within the same culture. Scale bars:A–C, 30 μm; D, E, 20 μm.
Fig. 3.
Stem cell lines established from the adult SVZ–RE display steady expansion rates. Cells were grown in the simultaneous presence of EGF and FGF-2, and growth curves were obtained (see Materials and Methods). Data were interpolated using a linear regression model and best fitted the following equation:y = a + b_x, where_y is the estimated total number of cells (in log scale),x is the time (DIV), a is the intercept value, and b is the slope. The values of_b_ ± SE are shown in the insets in_A–C_. For D–F, in which the growth curves of early and late cultures from SVZ, RE1, and RE2 are compared, refer to b values shown in A and_B_ for early and late cultures, respectively (A, B). The SVZ- and RE1-derived cell lines had closely similar expansion rates, whereas the rate for RE2-derived cell lines was slower, both for early (A) and late cultures (B). The expansion rates of RE1- and RE2-derived clonal cell lines (B5.14 and A7.16, respectively; C) and of the cell line established from surgically removed OB tissue (A01-RE2; A) closely matched those observed in bulk cultures established from the corresponding regions (A, B). Extensive subcultivation (up to 6 months in vitro) did not affect the growth characteristics of the SVZ- (D), RE1- (E), or RE2-derived (F) stem cell lines. Growth curves (early and late passages) were generated from each of three independent cultures, all of which yielded similar results. The growth curves presented are from one among the three independent cultures. The slope values were compared using a t test followed by a Bonferroni post hoc test. **Significantly different from SVZ, RE1, and RE1/B5.14; p < 0.05.
Fig. 4.
RE1 stem cells produce higher numbers of oligodendrocytes. SVZ-, RE1-, and RE2-derived stem cells (passages 5–23) were induced to differentiate by removal of GFs. The figure shows representative fields of stem cell-derived cultures from these regions after 6 d of differentiation and after indirect immunofluorescence with an anti-GalC antibody. Significantly less oligodendrocytes are found in SVZ (A) and RE2 stem cell progeny (B) as compared with RE1-derived cultures (C). See Table 2 for a detailed quantitative analysis. Scale bar, 20 μm.
Fig. 5.
RE-derived stem cells generate neuronal progeny displaying different neurotransmitter phenotype. Serially passaged (passages 5–23), SVZ-, and RE-derived stem cells were allowed to differentiate for 6 d by removal of GFs. Indirect immunofluorescence revealed the presence in RE1- and RE2-derived stem cell progeny, as well as in SVZ-derived cultures, of Tuj1-IR cells (A, C, E) that were IR for GABA (B), glutamic acid (D), and ChAT (F). This figure shows representative microphotograph of RE1-derived cells. Scale bars: A–D, 20 μm (shown in A); E,F, 20 μm (shown in E).
Similar articles
- Neurogenesis in the subventricular zone and rostral migratory stream of the neonatal and adult primate forebrain.
Pencea V, Bingaman KD, Freedman LJ, Luskin MB. Pencea V, et al. Exp Neurol. 2001 Nov;172(1):1-16. doi: 10.1006/exnr.2001.7768. Exp Neurol. 2001. PMID: 11681836 - Protein expression differs between neural progenitor cells from the adult rat brain subventricular zone and olfactory bulb.
Maurer MH, Feldmann RE Jr, Bürgers HF, Kuschinsky W. Maurer MH, et al. BMC Neurosci. 2008 Jan 16;9:7. doi: 10.1186/1471-2202-9-7. BMC Neurosci. 2008. PMID: 18197988 Free PMC article. - Postnatal neurogenesis and gliogenesis in the olfactory bulb from NG2-expressing progenitors of the subventricular zone.
Aguirre A, Gallo V. Aguirre A, et al. J Neurosci. 2004 Nov 17;24(46):10530-41. doi: 10.1523/JNEUROSCI.3572-04.2004. J Neurosci. 2004. PMID: 15548668 Free PMC article. - The heterogeneity of adult neural stem cells and the emerging complexity of their niche.
Alvarez-Buylla A, Kohwi M, Nguyen TM, Merkle FT. Alvarez-Buylla A, et al. Cold Spring Harb Symp Quant Biol. 2008;73:357-65. doi: 10.1101/sqb.2008.73.019. Epub 2008 Nov 6. Cold Spring Harb Symp Quant Biol. 2008. PMID: 19022766 Review. - Origin and function of olfactory bulb interneuron diversity.
Lledo PM, Merkle FT, Alvarez-Buylla A. Lledo PM, et al. Trends Neurosci. 2008 Aug;31(8):392-400. doi: 10.1016/j.tins.2008.05.006. Epub 2008 Jul 5. Trends Neurosci. 2008. PMID: 18603310 Free PMC article. Review.
Cited by
- EGF-induced expansion of migratory cells in the rostral migratory stream.
Lindberg OR, Persson A, Brederlau A, Shabro A, Kuhn HG. Lindberg OR, et al. PLoS One. 2012;7(9):e46380. doi: 10.1371/journal.pone.0046380. Epub 2012 Sep 28. PLoS One. 2012. PMID: 23029503 Free PMC article. - Cell therapy for multiple sclerosis.
Ben-Hur T. Ben-Hur T. Neurotherapeutics. 2011 Oct;8(4):625-42. doi: 10.1007/s13311-011-0073-x. Neurotherapeutics. 2011. PMID: 21904787 Free PMC article. Review. - Persistence of psychosine in brain lipid rafts is a limiting factor in the therapeutic recovery of a mouse model for Krabbe disease.
White AB, Galbiati F, Givogri MI, Lopez Rosas A, Qiu X, van Breemen R, Bongarzone ER. White AB, et al. J Neurosci Res. 2011 Mar;89(3):352-64. doi: 10.1002/jnr.22564. Epub 2010 Dec 29. J Neurosci Res. 2011. PMID: 21259322 Free PMC article. - Purinergic Signaling Pathway in Human Olfactory Neuronal Precursor Cells.
Solís-Chagoyán H, Flores-Soto E, Valdés-Tovar M, Cercós MG, Calixto E, Montaño LM, Barajas-López C, Sommer B, Aquino-Gálvez A, Trueta C, Benítez-King GA. Solís-Chagoyán H, et al. Stem Cells Int. 2019 Apr 2;2019:2728786. doi: 10.1155/2019/2728786. eCollection 2019. Stem Cells Int. 2019. PMID: 31065271 Free PMC article. - Insulin biosynthesis in neuronal progenitors derived from adult hippocampus and the olfactory bulb.
Kuwabara T, Kagalwala MN, Onuma Y, Ito Y, Warashina M, Terashima K, Sanosaka T, Nakashima K, Gage FH, Asashima M. Kuwabara T, et al. EMBO Mol Med. 2011 Dec;3(12):742-54. doi: 10.1002/emmm.201100177. Epub 2011 Oct 10. EMBO Mol Med. 2011. PMID: 21984534 Free PMC article.
References
- Altman J. Autoradiographic and histological studies of postnatal neurogenesis. IV. Cell proliferation and migration in the anterior forebrain, with special reference to persisting neurogenesis in the olfactory bulb. J Comp Neurol. 1969;137:433–458. - PubMed
- Altman J, Das GD. Autoradiographic and histological evidence of postnatal hippocampal neurogenesis in rat. J Comp Neurol. 1965;124:319–335. - PubMed
- Craig CD, D'Sa R, Morshead CM, Roach A, van der Kooy D. Migrational analysis of the constitutively proliferating subependymal population in the adult mouse forebrain. Neuroscience. 1999;93:1197–1206. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous