Selective vulnerability of late oligodendrocyte progenitors to hypoxia-ischemia - PubMed (original) (raw)
Selective vulnerability of late oligodendrocyte progenitors to hypoxia-ischemia
Stephen A Back et al. J Neurosci. 2002.
Abstract
In the premature infant, hypoxic-ischemic damage to the cerebral white matter [periventricular leukomalacia (PVL)] is a common and leading cause of brain injury that often results in chronic neurologic disability from cerebral palsy. The cellular basis for the propensity of white matter injury to occur in the developing brain and the greater resistance of the adult white matter to similar injury remains unknown. By using a neonatal rat model of hypoxic-ischemic injury, we found that the mechanism of perinatal white matter injury involved maturation-dependent vulnerability in the oligodendroctye (OL) lineage. The timing of appearance of late OL progenitors was the major developmental factor that accounted for the susceptibility of the neonatal white matter to injury. Late OL progenitors were the major OL lineage stage killed by apoptosis, whereas early OL progenitors and more mature OLs were highly resistant. The density of pyknotic late OL progenitors was significantly increased in the ischemic hemisphere (67 +/- 31 cells/mm2) versus the control hemisphere (2.2 +/- 0.4 cells/mm2; mean +/- SEM; p = 0.05), which resulted in the death of 72 +/- 6% of this OL stage. Surviving late OL progenitors displayed a reactive response in which an increase in cell density was accompanied by accelerated maturation to a P27/kip1-positive oligodendrocyte. Because we showed recently that late OL progenitors populate human cerebral white matter during the high risk period for PVL (Back et al., 2001), maturation-dependent vulnerability of OL progenitors to hypoxia-ischemia may underlie the selective vulnerability to PVL of the white matter in the premature infant.
Figures
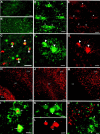
Fig. 1.
Susceptibility of late OL progenitors to H-I at P2. A, The four successive stages in the OL lineage are depicted at the top. Shown at the _bottom_are the markers that were applied to define each stage.B, The normal low-power distribution of O4-labeled cells in the corpus callosum in the control hemisphere. C, The ischemic corpus callosum contralateral to that in _B_contains numerous pyknotic O4-labeled cells (arrowheads). Adjacent to the infarct (bottom left) are numerous intensely labeled cells (arrows). These apparent reactive cells are discussed in the context of Figure 4. D, Pyknotic cells at various stages of degeneration. A halo of degenerating processes surrounds one pyknotic cell (arrow). Many pyknotic cells (arrowheads) at a more advanced stage of degeneration had no discernible processes. E, High-power detail of pyknotic OL progenitors (arrowheads). Typical morphology of a control cell (inset). F, The density (cells per square millimeter) of pyknotic late OL progenitors in the ischemic (Ipsilateral) hemisphere was significantly increased relative to the control (Contralateral) hemisphere (*p = 0.05; unpaired Student's _t_test). G, One week after H-I at P2, the area of the ischemic (Ipsilateral) corpus callosum was significantly decreased by ∼20% (*p = 0.0004; unpaired Student's t test). Scale bars:B, C, 100 μm; D, 50 μm; E and inset, 25 μm.
Fig. 2.
Pyknotic late OL progenitors label for markers of cell death. Numerous TUNEL-positive nuclei were detected in the ischemic corpus callosum (B) relative to control (A). C, Pyknotic O4 antibody-labeled cells (red, arrowheads) had TUNEL-positive nuclei (green).D, E, O4 antibody-labeled progenitors in the nonischemic hemisphere (D,arrowheads) displayed a punctate distribution of cytochrome c immunoreactivity in the soma and proximal processes (E). F,G, O4 antibody-labeled pyknotic OL progenitors (F, arrowheads) displayed a diffuse cytoplasmic distribution of cytochrome _c_immunoreactivity (G). H, Numerous cells immunoreactive for CM1, an antibody against activated caspase-3, were visualized in the ischemic cerebral cortex (CTX) and caudate–putamen (CPu), but few were visualized in the corpus callosum (CC).I, Pyknotic O4-labeled cells at different stages of degeneration (green, arrows) were CM 1 immunoreactive. J, Numerous cells immunoreactive for fractin were visualized in the ischemic CTX and CC. Note that the CPu is not shown in this higher-power photomicrograph.K, L, Pyknotic O4-labeled cells (K, arrowheads) labeled for fractin (L). M, Numerous cells immunoreactive for the p120 antibody against a caspase cleavage product of spectrin were visualized in the ischemic CTX but were not detected in the corpus callosum. N, Higher-power detail of the junction between the cerebral cortex and the corpus callosum demonstrates that pyknotic O4 antibody-labeled cells (green, arrows) were not p120 antibody immunoreactive, in contrast to numerous apparent cortical neurons (red). Scale bars: A,B, 100 μm; C, 20 μm;D, E, 10 μm; F,G, 7 μm; H, 100 μm; I, 10 μm; J, 50 μm; K, L, 7 μm; M, 60 μm; N, 30 μm.
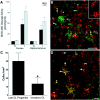
Fig. 3.
Maturation-dependent differences in OL lineage susceptibility are seen at P2 and P7. A, Injury to the ischemic (I) cerebral cortex and hippocampus at P2 and P7 was compared with control (C) by assay of tissue homogenates for DEVD-amino methyl coumarin cleavage reflecting activated caspase-3-like activity. Animals exposed to 6% hypoxia for 4 h at P2 had a similar increase in enzyme activity in the ischemic hemisphere compared with animals exposed for 2.5 h to 8% hypoxia at P7.B, Numerous reactive NG2-positive early OL progenitors (red, arrowheads and_inset_) in an ischemic lesion from a P2 animal overlapped in distribution with pyknotic late OL progenitors (green, short arrows). Note the apparent reactive OL (long arrow) that was resistant to H-I. C, After H-I at P7, the density (cells per square millimeter) of pyknotic late OL progenitors in the cerebral cortex was significantly greater than that of pyknotic immature OLs in the underlying corpus callosum (*p = 0.04; unpaired Student's t test). D, An ischemic lesion from a P2 animal was double labeled with O4 (red) and O1 (green) antibodies. Most pyknotic cells were O4+O1− late OL progenitors (red,arrows), and few pyknotic immature OLs were visualized (yellow, arrowheads). Note the double-labeled reactive-appearing OLs (yellow,long arrows) that appeared resistant to H-I. Scale bars:B, D, 50 μm.
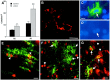
Fig. 4.
H-I triggers the proliferation of reactive OLs.A, By 24 h after H-I at P2 and P7, a significant increase in the density of O4+O1+ reactive OLs (*_p_= 0.04; **p = 0.02) occurred in the area adjacent to the ischemic lesion. B, Reactive OLs typically were visualized in clusters of two or more cells. C, A reactive OL in the apparent process of mitosis has a dividing nucleus (D) that was labeled with Hoechst 33324.E, Immature-appearing late OL progenitors in the ischemic penumbra at P8 (arrowheads) labeled for the O4 antibody (green) and the nuclear-associated cell proliferation marker MIB-5 (red). F, Mature-appearing reactive OLs (arrows) at P3 did not show nuclear-labeling with MIB-5 (arrowheads).G, Reactive OLs at P8 labeled with the O1 antibody (green, arrows) also labeled with an antibody against p27 that stained the nuclei of these and other cells (arrowheads). Scale bars:B_–_D, 10 μm; E,G, 15 μm; F, 20 μm.
Similar articles
- Late oligodendrocyte progenitors coincide with the developmental window of vulnerability for human perinatal white matter injury.
Back SA, Luo NL, Borenstein NS, Levine JM, Volpe JJ, Kinney HC. Back SA, et al. J Neurosci. 2001 Feb 15;21(4):1302-12. doi: 10.1523/JNEUROSCI.21-04-01302.2001. J Neurosci. 2001. PMID: 11160401 Free PMC article. - Arrested oligodendrocyte lineage maturation in chronic perinatal white matter injury.
Segovia KN, McClure M, Moravec M, Luo NL, Wan Y, Gong X, Riddle A, Craig A, Struve J, Sherman LS, Back SA. Segovia KN, et al. Ann Neurol. 2008 Apr;63(4):520-30. doi: 10.1002/ana.21359. Ann Neurol. 2008. PMID: 18393269 Free PMC article. - Hypoxic-ischemic injury results in acute disruption of myelin gene expression and death of oligodendroglial precursors in neonatal mice.
Skoff RP, Bessert DA, Barks JD, Song D, Cerghet M, Silverstein FS. Skoff RP, et al. Int J Dev Neurosci. 2001 Apr;19(2):197-208. doi: 10.1016/s0736-5748(00)00075-7. Int J Dev Neurosci. 2001. PMID: 11255033 - Perinatal white matter injury: the changing spectrum of pathology and emerging insights into pathogenetic mechanisms.
Back SA. Back SA. Ment Retard Dev Disabil Res Rev. 2006;12(2):129-40. doi: 10.1002/mrdd.20107. Ment Retard Dev Disabil Res Rev. 2006. PMID: 16807910 Review. - Neurobiology of periventricular leukomalacia in the premature infant.
Volpe JJ. Volpe JJ. Pediatr Res. 2001 Nov;50(5):553-62. doi: 10.1203/00006450-200111000-00003. Pediatr Res. 2001. PMID: 11641446 Review.
Cited by
- What are the Best Animal Models for Testing Early Intervention in Cerebral Palsy?
Clowry GJ, Basuodan R, Chan F. Clowry GJ, et al. Front Neurol. 2014 Dec 4;5:258. doi: 10.3389/fneur.2014.00258. eCollection 2014. Front Neurol. 2014. PMID: 25538677 Free PMC article. Review. - Spectrum of short- and long-term brain pathology and long-term behavioral deficits in male repeated hypoxic rats closely resembling human extreme prematurity.
Oorschot DE, Voss L, Covey MV, Goddard L, Huang W, Birchall P, Bilkey DK, Kohe SE. Oorschot DE, et al. J Neurosci. 2013 Jul 17;33(29):11863-77. doi: 10.1523/JNEUROSCI.0342-12.2013. J Neurosci. 2013. PMID: 23864676 Free PMC article. - Dual isolation of primary neurons and oligodendrocytes from guinea pig frontal cortex.
Moloney RA, Pavy CL, Kahl RGS, Palliser HK, Hirst JJ, Shaw JC. Moloney RA, et al. Front Cell Neurosci. 2024 Jan 10;17:1298685. doi: 10.3389/fncel.2023.1298685. eCollection 2023. Front Cell Neurosci. 2024. PMID: 38269115 Free PMC article. - Atoh1 mediated disturbance of neuronal maturation by perinatal hypoxia induces cognitive deficits.
Cai XY, Ma SY, Tang MH, Hu L, Wu KD, Zhang Z, Zhang YQ, Lin Y, Patel N, Yang ZC, Mo XM. Cai XY, et al. Commun Biol. 2024 Sep 11;7(1):1121. doi: 10.1038/s42003-024-06846-7. Commun Biol. 2024. PMID: 39261625 Free PMC article. - Timing of appearance of late oligodendrocyte progenitors coincides with enhanced susceptibility of preterm rabbit cerebral white matter to hypoxia-ischemia.
Buser JR, Segovia KN, Dean JM, Nelson K, Beardsley D, Gong X, Luo NL, Ren J, Wan Y, Riddle A, McClure MM, Ji X, Derrick M, Hohimer AR, Back SA, Tan S. Buser JR, et al. J Cereb Blood Flow Metab. 2010 May;30(5):1053-65. doi: 10.1038/jcbfm.2009.286. Epub 2010 Jan 13. J Cereb Blood Flow Metab. 2010. PMID: 20068573 Free PMC article.
References
- Back SA, Volpe JJ. Cellular and molecular pathogenesis of periventricular white matter injury. MRDD Res Rev. 1997;3:96–107.
- Back SA, Volpe JJ. Approaches to the study of diseases involving oligodendroglial death. In: Koliatsos V, Ratan R, editors. Cell death and diseases of the nervous system. Humana; Totowa, NJ: 1999. pp. 401–428.
Publication types
MeSH terms
Grants and funding
- P30 HD 33703/HD/NICHD NIH HHS/United States
- K12 HD033703/HD/NICHD NIH HHS/United States
- NS01855/NS/NINDS NIH HHS/United States
- NS41343/NS/NINDS NIH HHS/United States
- K02 NS041343/NS/NINDS NIH HHS/United States
- NS35902/NS/NINDS NIH HHS/United States
- P50 NS035902/NS/NINDS NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous