Antagonistic effects of Rnd1 and RhoD GTPases regulate receptor activity in Semaphorin 3A-induced cytoskeletal collapse - PubMed (original) (raw)
Antagonistic effects of Rnd1 and RhoD GTPases regulate receptor activity in Semaphorin 3A-induced cytoskeletal collapse
Silvio M Zanata et al. J Neurosci. 2002.
Abstract
The semaphorins are a large protein family that is involved in the patterning of neuronal connections in the developing nervous system of both vertebrates and invertebrates. The chemorepulsive axon guidance signal Semaphorin 3A (Sema3A) induces the depolymerization of actin filaments and the collapse of sensory growth cones by activating a receptor complex that contains a plexin as the signal-transducing subunit. Here we show that, of a large number of GTPases tested, only Rnd1 and RhoD bind the cytoplasmic domain of Plexin-A1. Recruitment of active Rnd1 is sufficient to trigger signaling by Plexin-A1, even in the absence of Sema3A, and initiates cytoskeletal collapse by activating its cytoplasmic domain. RhoD, in contrast, blocks Plexin-A1 activation by Rnd1 and repulsion of sympathetic axons by Sema3A. Thus, the antagonism of two GTPases regulates the activity of the Sema3A receptor, and activation by Rnd1 appears to be an essential step in signaling by Plexin-A1.
Figures
Fig. 1.
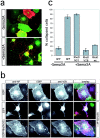
Interaction between Plexin-A1 and Rho-like GTPases. a, GST pull-down assays were performed with lysates of HEK 293T cells transfected with the expression vector pBK-VSV-Plexin-A1. Lysates were incubated with GST-Rnd1, -Rnd2, -Rac1, -RhoD, or -RhoG bound to glutathione-Sepharose beads and preloaded with GTPγS as indicated. Bound proteins were analyzed by Western blot using an anti-VSV or anti-GST antibody. Plexin-A1 specifically bound to Rnd1 and RhoD. b, GST-RhoD was preincubated with GTPγS or GDP as indicated and incubated with lysates of HEK 293T cells transfected with pBK-VSV-Plexin-A1. Preincubation with GDP substantially reduced the amount of bound RhoD.
Fig. 2.
RhoD blocks Sema3A-induced collapse of COS-7 cells. a, COS-7 cells were transfected with the expression vectors pBK-VSV-PlexinA1, pBK-HA-Nrp-1, and pEGFP and incubated with medium (−Sema3A) or with medium containing 0.4 n
m
AP-Sema3A (+Sema3A) for 1 hr at 37°C. Cells were fixed and processed for immunofluorescence without permeabilization. The expression of recombinant proteins was visualized by EGFP-fluorescence or indirect immunofluorescence using an anti-VSV antibody. b, COS-7 cells were transfected with the expression vectors pBK-VSV-PlexinA1, pBK-HA-Nrp-1, and pEGFP or pEGFP-RhoDV26 as indicated and incubated with medium (−) or with medium containing 0.4 n
m
AP-Sema3A (+AP-Sema3A) for 1 hr at 37°C. Bound AP-Sema3A was revealed using an anti-alkaline phosphatase antibody (red). The expression of recombinant proteins was visualized by EGFP-fluorescence (green) or indirect immunofluorescence using an anti-VSV antibody (blue). c, The number of cells collapsed in response to Sema3A was determined according to published criteria (Takahashi et al., 1999). The percentage of collapsed cells (gray bars) is displayed (n = 4; 200–300 cells counted per experiment). wt, Wild type.
Fig. 3.
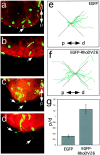
RhoD blocks repulsion of sympathetic axons by Sema3A. Explanted ganglia were transfected by particle-mediated gene transfer with a mixture of ptauEGFP and pEGFP (a,b, e) or pBK-EGFP-RhoDV26 (c, d, f), cultured together with Sema3A-expressing cell aggregates and analyzed after 24 hr of culture by following the trajectories of EGFP-positive axons (arrows). Cell aggregates are located outside the field of view (proximal is to the bottom). Staining with phalloidin–rhodamine confirmed that no EGFP-negative axons were present in the proximal quadrant. Representative images of transfected ganglia are shown. e, f, A camera lucida representation of tauEGFP-positive axons extending into proximal (p) and distal (d) quadrants is shown. Axonal trajectories were recorded from neurons located at the proximal edge of explanted ganglia facing the Sema3A-secreting cell aggregate (n = 6). Axons extending into the lateral quadrants are not informative for their Sema3A sensitivity and were not included in our analysis.g, EGFP-positive axons extending toward (p) or away (d) from the cell aggregates secreting Sema3A were counted, and the ratio of axons growing proximally to that growing distally (p/d ratio) was calculated. Whereas axons extended preferentially away from Sema3A-expressing cells in control transfections, cells transfected with the expression vector for RhoDV26 had axons that extended with similar probability either proximally or distally (n = 6, 20–40 ganglia per experiment).
Fig. 4.
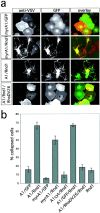
Interaction of Plexin-A1 and Rnd1 induces cell collapse. a, COS-7 cells were transfected with expression vectors for myr-myc-Plexin-A1 (myrA1), VSV-Plexin-A1 (A1), and EGFP, EGFP-Rnd1 (Rnd1), or EGFP-Rnd1 and EGFP-RhoDV26 as indicated and processed for indirect immunofluorescence. The expression of recombinant proteins was visualized by EGFP-fluorescence (green) and immunofluorescence using an anti-myc or anti-VSV antibody (red). Interaction of the Plexin-A1 cytoplasmic domain and Rnd1 induces cell collapse. Rnd1-induced cell collapse is blocked by coexpression of RhoDV26. b, The number of cells collapsed in response to Sema3A was determined, and the percentage of collapsed cells (gray bars) is displayed (n = 4, 200–300 cells counted per experiment). Only membrane-bound Plexin-A1 was able to induce cell collapse during coexpression with Rnd1. Coexpression of the cytoplasmic Plexin-A1 domain without myristoylation signal (A1cyt) with Rnd1 was not able to induce cell collapse.
Fig. 5.
Mutations disrupting Rnd1 interaction or the GAP-homology region inactivate Plexin-A1. Lysates of HEK 293T cells transfected with expression vectors for VSV-Plexin-A1, VSV-Plexin-A1P1 (a), or VSV-Plexin-A1P2 (b) were incubated with GST-Rnd1, -RhoD, or -Rac3 preloaded with GTPγS as indicated, and bound proteins were analyzed by Western blot using an anti-VSV or anti-GST antibody. Plexin-A1P1 but not Plexin-A1P2 bound to Rnd1. c, COS-7 cells were transfected with expression for Nrp-1, EGFP, and VSV-PlexinA1, VSV-PlexinA1P1, or VSV-PlexinA1P2 and incubated with medium containing 0.4 n
m
AP-Sema3A (+Sema3A) for 1 hr at 37°C. Cells were fixed and processed for immunofluorescence, and the number of cells collapsed in response to Sema3A was determined. The percentage of collapsed cells (gray bars) is displayed (n = 4, 200–300 cells counted per experiment). d, COS-7 cells were transfected with expression vectors for VSV-PlexinA1, VSV-PlexinA1P2, VSV-PlexinA1R12, and EGFP-Rnd1 or EGFP as indicated and were processed for immunofluorescence, and the percentage of collapsed cells (gray bars) was determined (n = 4, 200–300 cells counted per experiment). The mutants Plexin-A1P2 and Plexin-A1R12 were no longer able to induce cell collapse during coexpression with Rnd1.
Fig. 6.
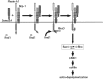
Plexin-A1, RhoD, and Rnd1 act like a logical AND gate. Sema3A binding to a receptor containing Nrp-1 as the ligand binding and Plexin-A1 as the signal transducing subunit triggers recruitment of Rnd1. Rnd1 has low intrinsic GTPase activity and is considered to be constitutively GTP bound. Interaction of Plexin-A1 and Rnd1 results in an activation of Plexin-A1 and downstream signaling events that shift the balance of Rac and Rho activity toward actin depolymerization. This process is blocked by interaction of Plexin-A1 with RhoD. The molecular components that link Rac and Rho to active Plexin-A1 are presently unknown.
Similar articles
- FARP2 triggers signals for Sema3A-mediated axonal repulsion.
Toyofuku T, Yoshida J, Sugimoto T, Zhang H, Kumanogoh A, Hori M, Kikutani H. Toyofuku T, et al. Nat Neurosci. 2005 Dec;8(12):1712-9. doi: 10.1038/nn1596. Epub 2005 Nov 13. Nat Neurosci. 2005. PMID: 16286926 - Direct interaction of Rnd1 with Plexin-B1 regulates PDZ-RhoGEF-mediated Rho activation by Plexin-B1 and induces cell contraction in COS-7 cells.
Oinuma I, Katoh H, Harada A, Negishi M. Oinuma I, et al. J Biol Chem. 2003 Jul 11;278(28):25671-7. doi: 10.1074/jbc.M303047200. Epub 2003 May 1. J Biol Chem. 2003. PMID: 12730235 - Plexin/neuropilin complexes mediate repulsion by the axonal guidance signal semaphorin 3A.
Rohm B, Ottemeyer A, Lohrum M, Püschel AW. Rohm B, et al. Mech Dev. 2000 May;93(1-2):95-104. doi: 10.1016/s0925-4773(00)00269-0. Mech Dev. 2000. PMID: 10781943 - Molecular basis of semaphorin-mediated axon guidance.
Nakamura F, Kalb RG, Strittmatter SM. Nakamura F, et al. J Neurobiol. 2000 Aug;44(2):219-29. doi: 10.1002/1097-4695(200008)44:2<219::aid-neu11>3.0.co;2-w. J Neurobiol. 2000. PMID: 10934324 Review. - GTPases in semaphorin signaling.
Püschel AW. Püschel AW. Adv Exp Med Biol. 2007;600:12-23. doi: 10.1007/978-0-387-70956-7_2. Adv Exp Med Biol. 2007. PMID: 17607943 Review.
Cited by
- Structural basis of Rnd1 binding to plexin Rho GTPase binding domains (RBDs).
Wang H, Hota PK, Tong Y, Li B, Shen L, Nedyalkova L, Borthakur S, Kim S, Tempel W, Buck M, Park HW. Wang H, et al. J Biol Chem. 2011 Jul 22;286(29):26093-106. doi: 10.1074/jbc.M110.197053. Epub 2011 May 24. J Biol Chem. 2011. PMID: 21610070 Free PMC article. - Semaphorin signals on the road to cancer invasion and metastasis.
Rizzolio S, Tamagnone L. Rizzolio S, et al. Cell Adh Migr. 2007 Apr-Jun;1(2):62-8. doi: 10.4161/cam.1.2.4570. Epub 2007 Apr 13. Cell Adh Migr. 2007. PMID: 19329883 Free PMC article. Review. - Control of cellular motility by neuropilin-mediated physical interactions.
Li X, Parker MW, Vander Kooi CW. Li X, et al. Biomol Concepts. 2014 May;5(2):157-66. doi: 10.1515/bmc-2013-0035. Biomol Concepts. 2014. PMID: 25018786 Free PMC article. Review. - Diverse functions for the semaphorin receptor PlexinD1 in development and disease.
Gay CM, Zygmunt T, Torres-Vázquez J. Gay CM, et al. Dev Biol. 2011 Jan 1;349(1):1-19. doi: 10.1016/j.ydbio.2010.09.008. Epub 2010 Sep 27. Dev Biol. 2011. PMID: 20880496 Free PMC article. Review. - Semaphorin 4D signaling requires the recruitment of phospholipase C gamma into the plexin-B1 receptor complex.
Swiercz JM, Worzfeld T, Offermanns S. Swiercz JM, et al. Mol Cell Biol. 2009 Dec;29(23):6321-34. doi: 10.1128/MCB.00103-09. Epub 2009 Oct 5. Mol Cell Biol. 2009. PMID: 19805522 Free PMC article.
References
- Aizawa H, Wakatsuki S, Ishii A, Moriyama K, Sasaki Y, Ohashi K, Sekine-Aizawa Y, Sehara-Fujisawa A, Mizuno K, Goshima Y, Yahara I. Phosphorylation of cofilin by LIM-kinase is necessary for semaphorin 3A-induced growth cone collapse. Nat Neurosci. 2001;4:367–373. - PubMed
- Bagnard D, Lohrum M, Uziel D, Püschel AW, Bolz J. Semaphorins act as attractive and repulsive guidance signals during the development of cortical projections. Development. 1998;125:5043–5053. - PubMed
- Castellani V, Chedotal A, Schachner M, Faivre-Sarrailh C, Rougon G. Analysis of the L1-deficient mouse phenotype reveals cross-talk between Sema3A and L1 signaling pathways in axonal guidance. Neuron. 2000;27:237–249. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources