ERK MAP kinase activation in superficial spinal cord neurons induces prodynorphin and NK-1 upregulation and contributes to persistent inflammatory pain hypersensitivity - PubMed (original) (raw)
ERK MAP kinase activation in superficial spinal cord neurons induces prodynorphin and NK-1 upregulation and contributes to persistent inflammatory pain hypersensitivity
Ru-Rong Ji et al. J Neurosci. 2002.
Abstract
Activation of ERK (extracellular signal-regulated kinase) MAP (mitogen-activated protein) kinase in dorsal horn neurons of the spinal cord by peripheral noxious stimulation contributes to short-term pain hypersensitivity. We investigated ERK activation by peripheral inflammation and its involvement in regulating gene expression in the spinal cord and in contributing to inflammatory pain hypersensitivity. Injection of complete Freund's adjuvant (CFA) into a hindpaw produced a persistent inflammation and a sustained ERK activation in neurons in the superficial layers (laminae I-IIo) of the dorsal horn. CFA also induced an upregulation of prodynorphin and neurokinin-1 (NK-1) in dorsal horn neurons, which was suppressed by intrathecal delivery of the MEK (MAP kinase kinase) inhibitor U0126. CFA-induced phospho-ERK primarily colocalized with prodynorphin and NK-1 in superficial dorsal horn neurons. Although intrathecal injection of U0126 did not affect basal pain sensitivity, it did attenuate both the establishment and maintenance of persistent inflammatory heat and mechanical hypersensitivity. Activation of the ERK pathway in a subset of nociceptive spinal neurons contributes, therefore, to persistent pain hypersensitivity, possibly via transcriptional regulation of genes, such as prodynorphin and NK-1.
Figures
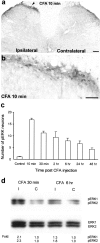
Fig. 1.
CFA induces a sustained activation of ERK.a, A low-magnification image showing induction of ERK phosphorylation in laminae I–IIo neurons of the ipsilateral spinal cord (indicated with an arrowhead) 10 min after CFA injection into a hindpaw. Scale bar, 200 μm. b, A high-magnification image of a, showing ERK activation in the medial superficial dorsal horn of the ipsilateral spinal cord 10 min after CFA injection. Scale bar, 50 μm. c, Time course of pERK induction after CFA administration measured by the number of pERK-positive neurons in the superficial (I–IIo) layers of the ipsilateral dorsal horn. Data are represented as mean ± SEM (n = 3). d, Western blot showing increased ERK phosphorylation of both ERK1 (44 kDa) and ERK2 (42 kDa) in the ipsilateral (I) dorsal horn compared with contralateral (C) side, 30 min and 6 hr after CFA injection. The bottom panel indicates levels of total ERK1 and ERK2, as loading controls. _Fold_represents comparative levels over the corresponding contralateral side after normalizing for loading.
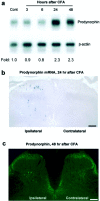
Fig. 2.
CFA induces prodynorphin upregulation in the dorsal horn. a, An RNase protection assay reveals an increase in prodynorphin mRNA in the ipsilateral dorsal horn 24 and 48 hr after CFA injection. Fold represents comparative levels over control after normalizing for loading. b,In situ hybridization indicates an increased expression of prodynorphin mRNA in ipsilateral superficial and deep dorsal horn neurons 24 hr after CFA. Scale bar, 50 μm. c, Increased number of prodynorphin-immunoreactive neurons was induced in the ipsilateral superficial and deep dorsal horn by CFA injection at 48 hr. Scale bar, 50 μm.
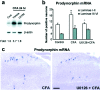
Fig. 3.
ERK activation regulates prodynorphin expression.a, Partial suppression of the CFA-induced increase in prodynorphin mRNA in the dorsal horn at 24 hr by U0126 (1 μg, intrathecally injected 30 min before and 6 hr after CFA).Fold represents comparative levels over control after normalizing for loading. b, Quantification of prodynorphin mRNA-positive neurons in laminae I–II and III–VI of the ipsilateral dorsal horn 24 hr after CFA injection. *p < 0.001, compared with control; +p < 0.001, compared with CFA (n = 4). c, _In situ_hybridization showing an inhibition of the CFA-induced increase in prodynorphin mRNA-labeled neurons in the superficial dorsal horn by U0126 24 hr after CFA injection. Scale bar, 50 μm.
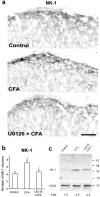
Fig. 4.
ERK activation regulates NK-1 expression.a, Suppression of the CFA-induced increase in NK-1 immunoreactivity in the medial superficial dorsal horn at 48 hr by U0126 delivered via an osmotic pump. Scale bar, 50 μm.b, Quantification of the numbers of NK-1 neurons in laminae I–IIo of the ipsilateral dorsal horn 48 hr after CFA injection. *p < 0.001, compared with control; +p < 0.001, compared with CFA (n = 5). c, Western blot indicates that the CFA-induced NK-1 increase in the dorsal horn at 24 hr is inhibited by U0126 (1 μg, intrathecally injected 30 min before and 6 hr after CFA injection). CREB, a constitutively expressed protein, was used as a loading control. Fold represents comparative levels over control after normalizing for loading.
Fig. 5.
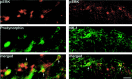
ERK is activated in a subset of prodynorphin- and NK-1-expressing neurons. pERK (red) is primarily colocalized with prodynorphin (green; left) and NK-1 (green; right) in the medial superficial dorsal horn 24 hr after CFA injection.Arrows indicate double-labeled neurons. Scale bar, 20 μm.
Fig. 6.
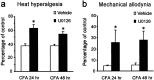
Sustained infusion of an MEK inhibitor reduces CFA-induced inflammatory pain. The MEK inhibitor U0126 delivered by osmotic pump (0.5 μg · μl−1 · hr−1) before CFA injection reduces thermal hyperalgesia (a) and mechanical allodynia (b) 24 and 48 hr after CFA injection. These were measured by paw-withdrawal latency and paw-withdrawal threshold, respectively, and expressed as percentage of pre-CFA baseline measurements of vehicle control (50% DMSO). *p < 0.01, compared with corresponding vehicle control (n = 8).
Fig. 7.
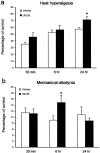
Post-treatment with an MEK inhibitor has a delayed effect on inflammatory pain. U0126 (1 μg) or vehicle (10% DMSO) was intrathecally administered 24 hr after CFA injection. Heat hyperalgesia (a) and mechanical allodynia (b) were tested 30 min, 6 hr, and 24 hr after the administration of the U0126. *p < 0.05, compared with corresponding vehicle control (n = 10). The data are expressed as percentage of pre-CFA baseline measurements of vehicle control.
Similar articles
- Spinal NF-kB activation induces COX-2 upregulation and contributes to inflammatory pain hypersensitivity.
Lee KM, Kang BS, Lee HL, Son SJ, Hwang SH, Kim DS, Park JS, Cho HJ. Lee KM, et al. Eur J Neurosci. 2004 Jun;19(12):3375-81. doi: 10.1111/j.0953-816X.2004.03441.x. Eur J Neurosci. 2004. PMID: 15217394 - ERK MAP kinase activation in spinal cord regulates phosphorylation of Cdk5 at serine 159 and contributes to peripheral inflammation induced pain/hypersensitivity.
Zhang X, Zhang H, Shao H, Xue Q, Yu B. Zhang X, et al. PLoS One. 2014 Jan 31;9(1):e87788. doi: 10.1371/journal.pone.0087788. eCollection 2014. PLoS One. 2014. PMID: 24498195 Free PMC article. - Peripheral and central mechanisms of inflammatory pain, with emphasis on MAP kinases.
Ji RR. Ji RR. Curr Drug Targets Inflamm Allergy. 2004 Sep;3(3):299-303. doi: 10.2174/1568010043343804. Curr Drug Targets Inflamm Allergy. 2004. PMID: 15379598 Review. - BDNF in sensory neurons and chronic pain.
Obata K, Noguchi K. Obata K, et al. Neurosci Res. 2006 May;55(1):1-10. doi: 10.1016/j.neures.2006.01.005. Epub 2006 Mar 3. Neurosci Res. 2006. PMID: 16516994 Review.
Cited by
- Metabotropic glutamate receptor 5 contributes to inflammatory tongue pain via extracellular signal-regulated kinase signaling in the trigeminal spinal subnucleus caudalis and upper cervical spinal cord.
Liu MG, Matsuura S, Shinoda M, Honda K, Suzuki I, Shibuta K, Tamagawa T, Katagiri A, Kiyomoto M, Ohara K, Furukawa A, Urata K, Iwata K. Liu MG, et al. J Neuroinflammation. 2012 Nov 27;9:258. doi: 10.1186/1742-2094-9-258. J Neuroinflammation. 2012. PMID: 23181395 Free PMC article. - Minocycline markedly reduces acute visceral nociception via inhibiting neuronal ERK phosphorylation.
Cho IH, Lee MJ, Jang M, Gwak NG, Lee KY, Jung HS. Cho IH, et al. Mol Pain. 2012 Feb 24;8:13. doi: 10.1186/1744-8069-8-13. Mol Pain. 2012. PMID: 22364340 Free PMC article. - Calcium calmodulin-stimulated adenylyl cyclases contribute to activation of extracellular signal-regulated kinase in spinal dorsal horn neurons in adult rats and mice.
Wei F, Vadakkan KI, Toyoda H, Wu LJ, Zhao MG, Xu H, Shum FW, Jia YH, Zhuo M. Wei F, et al. J Neurosci. 2006 Jan 18;26(3):851-61. doi: 10.1523/JNEUROSCI.3292-05.2006. J Neurosci. 2006. PMID: 16421305 Free PMC article. - p38 mitogen-activated protein kinase inhibitor SB203580 reverses the antianalgesia induced by dextro-morphine or morphine in the mouse spinal cord.
Wu HE, Sun HS, Cheng CW, Tseng LF. Wu HE, et al. Eur J Pharmacol. 2006 Nov 21;550(1-3):91-4. doi: 10.1016/j.ejphar.2006.08.060. Epub 2006 Sep 8. Eur J Pharmacol. 2006. PMID: 17026985 Free PMC article. - Roles of the AMPA receptor subunit GluA1 but not GluA2 in synaptic potentiation and activation of ERK in the anterior cingulate cortex.
Toyoda H, Zhao MG, Ulzhöfer B, Wu LJ, Xu H, Seeburg PH, Sprengel R, Kuner R, Zhuo M. Toyoda H, et al. Mol Pain. 2009 Aug 10;5:46. doi: 10.1186/1744-8069-5-46. Mol Pain. 2009. PMID: 19664265 Free PMC article.
References
- Abbadie C, Brown JL, Mantyh PW, Basbaum AI. Spinal cord substance P receptor immunoreactivity increases in both inflammatory and nerve injury models of persistent pain. Neuroscience. 1996;70:201–209. - PubMed
- Anderson LE, Seybold VS. Phosphorylated cAMP response element binding protein increases in neurokinin-1 receptor-immunoreactive neurons in rat spinal cord in response to formalin-induced nociception. Neurosci Lett. 2000;283:29–32. - PubMed
- Atkins CM, Selcher JC, Petraitis JJ, Trzaskos JM, Sweatt JD. The MAPK cascade is required for mammalian associative learning. Nat Neurosci. 1998;1:602–609. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous