Reduced hippocampal neurogenesis in adult transgenic mice with chronic astrocytic production of interleukin-6 - PubMed (original) (raw)
Reduced hippocampal neurogenesis in adult transgenic mice with chronic astrocytic production of interleukin-6
Luc Vallières et al. J Neurosci. 2002.
Abstract
Postnatal neurogenesis can be modulated after brain injury, but the role of the attendant expression of inflammatory mediators in such responses remains to be determined. Here we report that transgenically directed production of interleukin-6 (IL-6) by astroglia decreased overall neurogenesis by 63% in the hippocampal dentate gyrus of young adult transgenic mice. The proliferation, survival, and differentiation of neural progenitor cells labeled with the thymidine analog bromodeoxyuridine were all reduced in the granule cell layer of these mice, whereas their distribution and gliogenesis appeared normal. These effects were not a consequence of general toxicity of the IL-6 transgene, because they were manifested in the absence of neuronal death and of major changes in glial cell number and morphology. These findings suggest that long-term exposure of the brain to proinflammatory mediators such as IL-6, as is seen in certain degenerative disorders and infections, can interfere with adult neurogenesis.
Figures
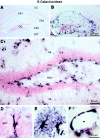
Fig. 1.
IL-6 transgene expression in the hippocampus revealed by β-gal immunohistochemistry. Brightfield photomicrographs of neutral red counterstained material show a lack of β-gal staining in the hippocampus of a wild-type mouse (A); β-gal staining in the hippocampus of an IL-6 transgenic mouse (B); higher magnification of β-gal staining in the dentate gyrus, showing many positive cells at the border between the granule cell layer and the hilus (C); a radial-like astrocyte within the granule cell layer expressing the IL-6 transgene (D); protoplasmic astrocytes of the gray matter (E); and blood vessel-associated astrocytes (F). CA1–CA3, Fields CA1–CA3 of Ammon's horn; CC, cerebral cortex;DG, dentate gyrus; GCL, granule cell layer; LDT, lateral dorsal thalamic nucleus;ML, molecular layer. The _asterisk_indicates lumen of blood vessel.
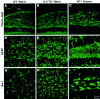
Fig. 2.
Neuronal integrity and gliosis in the dentate gyrus revealed by Fluoro-jade, GFAP, and Iba-1 histochemistry. Confocal laser scanning microscopic images of sections through the dentate gyrus show absence of Fluoro-jade staining in a wild-type (WT) mouse (A), absence of Fluoro-jade staining in an IL-6 transgenic (TG) mouse (B), Fluoro-jade-positive cells in the hilar region of a control mouse subjected to seizures (C), GFAP immunoreactivity in a WT mouse (D), GFAP immunostaining indicative of a mild astrogliosis in a TG mouse (E), hypertrophic reactive astrocytes in the hilus of a control mouse after seizures (F), Iba-1 immunoreactivity in a WT mouse (G), Iba-1 immunostaining indicating a moderate microgliosis in a TG mouse (H), and rounded, hypertrophied microglia in the hilus of a control mouse after seizures (I). GCL, Granule cell layer.
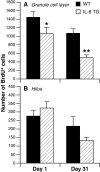
Fig. 3.
Effect of long-term IL-6 transgene expression on progenitor cell proliferation (day 1) and survival (day 31) in the granule cell layer of the dentate gyrus. A, Mean ± SEM of BrdU-labeled cells in the granule cell layer. B, Mean ± SEM of BrdU-labeled cells in the hilar region.TG, Transgenic; WT, wild-type. *p = 0.05; **p < 0.01.
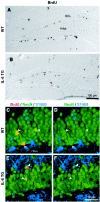
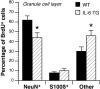
Fig. 4.
Progenitor cell distribution and phenotype in the dentate gyrus of wild-type and IL-6 transgenic mice. Differential interference contrast images show BrdU-positive cells in the dentate gyrus of wild-type (WT) mice (A) and IL-6 transgenic (TG) mice (B), both killed 1 d after the final BrdU injection. Merged confocal images show triple immunolabeling for BrdU (red), NeuN (green), and S100β (blue) in the granule cell layer of WT (C) and TG (E) mice killed 31 d after final BrdU injections. D, F, The same images as seen in C and E, respectively, but without BrdU labeling. GCL, Granule cell layer.Arrowheads indicate BrdU-positive cells double-labeled with NeuN. Arrows indicate BrdU-positive cells not labeled with NeuN.
Fig. 5.
Effect of long-term IL-6 transgene expression on progenitor differentiation in the granule cell layer 31 d after BrdU injection. Data are expressed as mean ± SEM of BrdU-labeled cells. TG, Transgenic; WT, wild-type. *p < 0.01.
Similar articles
- Astrocytes give rise to new neurons in the adult mammalian hippocampus.
Seri B, García-Verdugo JM, McEwen BS, Alvarez-Buylla A. Seri B, et al. J Neurosci. 2001 Sep 15;21(18):7153-60. doi: 10.1523/JNEUROSCI.21-18-07153.2001. J Neurosci. 2001. PMID: 11549726 Free PMC article. - Genetic influence on neurogenesis in the dentate gyrus of two strains of adult mice.
Schauwecker PE. Schauwecker PE. Brain Res. 2006 Nov 20;1120(1):83-92. doi: 10.1016/j.brainres.2006.08.086. Epub 2006 Sep 26. Brain Res. 2006. PMID: 16999941 - A transgenic marker for newly born granule cells in dentate gyrus.
Overstreet LS, Hentges ST, Bumaschny VF, de Souza FS, Smart JL, Santangelo AM, Low MJ, Westbrook GL, Rubinstein M. Overstreet LS, et al. J Neurosci. 2004 Mar 31;24(13):3251-9. doi: 10.1523/JNEUROSCI.5173-03.2004. J Neurosci. 2004. PMID: 15056704 Free PMC article. - Neurogenesis in adult mammals: some progress and problems.
Gould E, Gross CG. Gould E, et al. J Neurosci. 2002 Feb 1;22(3):619-23. doi: 10.1523/JNEUROSCI.22-03-00619.2002. J Neurosci. 2002. PMID: 11826089 Free PMC article. Review. No abstract available.
Cited by
- Endogenous CNTF mediates stroke-induced adult CNS neurogenesis in mice.
Kang SS, Keasey MP, Arnold SA, Reid R, Geralds J, Hagg T. Kang SS, et al. Neurobiol Dis. 2013 Jan;49:68-78. doi: 10.1016/j.nbd.2012.08.020. Epub 2012 Aug 31. Neurobiol Dis. 2013. PMID: 22960105 Free PMC article. - Cognitive dysfunction with aging and the role of inflammation.
Simen AA, Bordner KA, Martin MP, Moy LA, Barry LC. Simen AA, et al. Ther Adv Chronic Dis. 2011 May;2(3):175-95. doi: 10.1177/2040622311399145. Ther Adv Chronic Dis. 2011. PMID: 23251749 Free PMC article. - Interleukin-6 gene (IL-6): a possible role in brain morphology in the healthy adult brain.
Baune BT, Konrad C, Grotegerd D, Suslow T, Birosova E, Ohrmann P, Bauer J, Arolt V, Heindel W, Domschke K, Schöning S, Rauch AV, Uhlmann C, Kugel H, Dannlowski U. Baune BT, et al. J Neuroinflammation. 2012 Jul 6;9:125. doi: 10.1186/1742-2094-9-125. J Neuroinflammation. 2012. PMID: 22695063 Free PMC article. - From the Bush to the Brain: Preclinical Stages of Ethnobotanical Anti-Inflammatory and Neuroprotective Drug Discovery-An Australian Example.
Kumar P, Mathew S, Gamage R, Bodkin F, Doyle K, Rossetti I, Wagnon I, Zhou X, Raju R, Gyengesi E, Münch G. Kumar P, et al. Int J Mol Sci. 2023 Jul 4;24(13):11086. doi: 10.3390/ijms241311086. Int J Mol Sci. 2023. PMID: 37446262 Free PMC article. Review. - Hippocampal neuroprotection mediated by secretome of human mesenchymal stem cells against experimental stroke.
Asgari Taei A, Dargahi L, Khodabakhsh P, Kadivar M, Farahmandfar M. Asgari Taei A, et al. CNS Neurosci Ther. 2022 Sep;28(9):1425-1438. doi: 10.1111/cns.13886. Epub 2022 Jun 18. CNS Neurosci Ther. 2022. PMID: 35715988 Free PMC article.
References
- Abercrombie M. Estimation of nuclear population from microtome sections. Anat Rec. 1946;94:239–247. - PubMed
- Baranzini SE, Elfstrom C, Chang SY, Butunoi C, Murray R, Higuchi R, Oksenberg JR. Transcriptional analysis of multiple sclerosis brain lesions reveals a complex pattern of cytokine expression. J Immunol. 2000;165:6576–6582. - PubMed
- Bonni A, Sun Y, Nadal-Vicens M, Bhatt A, Frank DA, Rozovsky I, Stahl N, Yancopoulos GD, Greenberg ME. Regulation of gliogenesis in the central nervous system by the JAK-STAT signaling pathway. Science. 1997;278:477–483. - PubMed
- Brochu S, Olivier M, Rivest S. Neuronal activity and transcription of proinflammatory cytokines, IkappaBalpha, and iNOS in the mouse brain during acute endotoxemia and chronic infection with Trypanosoma brucei brucei. J Neurosci Res. 1999;57:801–816. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- NS21182/NS/NINDS NIH HHS/United States
- AGO6088/AG/NIA NIH HHS/United States
- AGO5131/AG/NIA NIH HHS/United States
- R37 NS021182/NS/NINDS NIH HHS/United States
- MH50426/MH/NIMH NIH HHS/United States
- R01 NS021182/NS/NINDS NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases