p62 Is a common component of cytoplasmic inclusions in protein aggregation diseases - PubMed (original) (raw)
p62 Is a common component of cytoplasmic inclusions in protein aggregation diseases
Kurt Zatloukal et al. Am J Pathol. 2002 Jan.
Abstract
Exposure of cells to stress, particularly oxidative stress, leads to misfolding of proteins and, if they are not refolded or degraded, to cytoplasmic protein aggregates. Protein aggregates are characteristic features of a variety of chronic toxic and degenerative diseases, such as Mallory bodies (MBs) in hepatocytes in alcoholic and non-alcoholic steatohepatitis, neurofibrillary tangles in neurons in Alzheimer's, and Lewy bodies in Parkinson's disease. Using 2D gel electrophoresis and mass spectrometry, we identified p62 as a novel MB component. p62 and cytokeratins (CKs) are major MB constituents; HSP 70, HSP 25, and ubiquitinated CKs are also present. These proteins characterize MBs as a prototype of disease-associated cytoplasmic inclusions generated by stress-induced protein misfolding. As revealed by transfection of tissue culture cells overexpressed p62 did not induce aggregation of regular CK filaments but selectively bound to misfolded and ubiquitinated CKs. The general role of p62 in the cellular response to misfolded proteins was substantiated by detection of p62 in other cytoplasmic inclusions, such as neurofibrillary tangles, Lewy bodies, Rosenthal fibers, intracytoplasmic hyaline bodies in hepatocellular carcinoma, and alpha1-antitrypsin aggregates. The presence of p62 along with other stress proteins and ubiquitin in cytoplasmic inclusions indicates deposition as aggregates as a third line of defense against misfolded proteins in addition to refolding and degradation.
Figures
Figure 1.
Analysis of MB components by MALDI-TOF mass spectrometry. a: Coomassie blue-stained 2D gel of MB proteins isolated from DDC-intoxicated mouse liver. Numbers indicate protein spots used for MALDI-TOF analysis. b: Summary of gel spot identifications using tryptic protein digests and MALDI-TOF mass spectrometry.
Figure 2.
P62 associated with MBs contains the epitope recognized by SMI 31. Coomassie blue-stained 2D gel of MB proteins isolated from DDC-intoxicated mouse liver (a) and the corresponding immunoblots with antibodies to p62 (c) and the SMI 31 (b) antibody.
Figure 3.
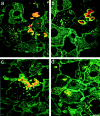
p62 and heat shock proteins are present in murine MBs. Double-label immunofluorescence microscopy using antibodies to CK (green) and the following antibodies to non-CK MB components (red): to p62 (a), to ubiquitin (b), to HSP 25 (c), and to HSP 70 (d). Scale bar, 10 μm.
Figure 4.
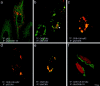
Interaction of p62 with cytokeratin is ubiquitin dependent. The interaction of p62 was studied with cell lines transiently cotransfected with different combinations of CK 8, ubiquitin, and p62 expression constructs. a: Double-label immunofluorescence microscopy (IF) with antibodies to CK 8 and 18 (green) and to p62 (red) of HepG2 cells transfected (TF) with p62. b: IF with antibodies to CK 8 (green) and p62 (red) of CHO-K1 cells transfected with CK 8 and ubiquitin. c: IF with antibodies to CK 8 (green) and to p62 (red) of CHO-K1 cells transfected with CK 8, ubiquitin, and p62. d: IF with antibodies to ubiquitin (green) and to p62 (red) of CHO-K1 cells transfected with CK 8, ubiquitin, and p62. e: IF with antibodies to CK 8 (green) and to ubiquitin (red) of CHO-K1 cell transfected with CK 8 and ubiquitin. f: IF with antibodies to CK 8 (green) and to ubiquitin (red) of CHO-K1 cells transfected with CK 8, CK 18, and ubiquitin. Scale bar, 10 μm.
Figure 5.
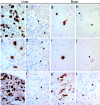
p62 is a common denominator of cytoplasmic inclusions in a variety of human diseases. Immunohistochemical detection of p62 in MBs in human alcoholic hepatitis (a), inclusions in α1-antitrypsin deficiency (c), intracytoplasmic hyaline bodies in hepatocellular carcinoma (e), neurofibrillary tangles in Alzheimer’s disease (g), Lewy bodies in Parkinson’s disease (i), and Rosenthal fibers in astrocytoma (k). For control of antibody specificity, immunoreactions were performed in parallel in the presence of the p62 peptide used for immunization resulting in complete inhibition of antibody binding (b, d, f, h, j, l). Arrowheads indicate examples of cytoplasmic inclusions in the corresponding tissues. Magnification, ×300.
Similar articles
- Interaction of stress proteins with misfolded keratins.
Janig E, Stumptner C, Fuchsbichler A, Denk H, Zatloukal K. Janig E, et al. Eur J Cell Biol. 2005 Mar;84(2-3):329-39. doi: 10.1016/j.ejcb.2004.12.018. Eur J Cell Biol. 2005. PMID: 15819411 - In vitro production of Mallory bodies and intracellular hyaline bodies: the central role of sequestosome 1/p62.
Stumptner C, Fuchsbichler A, Zatloukal K, Denk H. Stumptner C, et al. Hepatology. 2007 Sep;46(3):851-60. doi: 10.1002/hep.21744. Hepatology. 2007. PMID: 17685470 - Are the Mallory bodies and intracellular hyaline bodies in neoplastic and non-neoplastic hepatocytes related?
Denk H, Stumptner C, Fuchsbichler A, Müller T, Farr G, Müller W, Terracciano L, Zatloukal K. Denk H, et al. J Pathol. 2006 Apr;208(5):653-61. doi: 10.1002/path.1946. J Pathol. 2006. PMID: 16477590 - From Mallory to Mallory-Denk bodies: what, how and why?
Zatloukal K, French SW, Stumptner C, Strnad P, Harada M, Toivola DM, Cadrin M, Omary MB. Zatloukal K, et al. Exp Cell Res. 2007 Jun 10;313(10):2033-49. doi: 10.1016/j.yexcr.2007.04.024. Epub 2007 Apr 27. Exp Cell Res. 2007. PMID: 17531973 Review. - Emerging role of p62/sequestosome-1 in the pathogenesis of Alzheimer's disease.
Salminen A, Kaarniranta K, Haapasalo A, Hiltunen M, Soininen H, Alafuzoff I. Salminen A, et al. Prog Neurobiol. 2012 Jan;96(1):87-95. doi: 10.1016/j.pneurobio.2011.11.005. Epub 2011 Nov 22. Prog Neurobiol. 2012. PMID: 22138392 Review.
Cited by
- The CDT of Helicobacter hepaticus induces pro-survival autophagy and nucleoplasmic reticulum formation concentrating the RNA binding proteins UNR/CSDE1 and P62/SQSTM1.
He W, Azzi-Martin L, Velasco V, Lehours P, Dubus P, Djavaheri-Mergny M, Ménard A. He W, et al. PLoS Pathog. 2021 Mar 4;17(3):e1009320. doi: 10.1371/journal.ppat.1009320. eCollection 2021 Mar. PLoS Pathog. 2021. PMID: 33662035 Free PMC article. - Parkinsonian toxin-induced oxidative stress inhibits basal autophagy in astrocytes via NQO2/quinone oxidoreductase 2: Implications for neuroprotection.
Janda E, Lascala A, Carresi C, Parafati M, Aprigliano S, Russo V, Savoia C, Ziviani E, Musolino V, Morani F, Isidoro C, Mollace V. Janda E, et al. Autophagy. 2015;11(7):1063-80. doi: 10.1080/15548627.2015.1058683. Autophagy. 2015. PMID: 26046590 Free PMC article. - Autophagy is active in normal colon mucosa.
Groulx JF, Khalfaoui T, Benoit YD, Bernatchez G, Carrier JC, Basora N, Beaulieu JF. Groulx JF, et al. Autophagy. 2012 Jun;8(6):893-902. doi: 10.4161/auto.19738. Epub 2012 Jun 1. Autophagy. 2012. PMID: 22652752 Free PMC article. - Aggregability of the SQSTM1/p62-based aggresome-like induced structures determines the sensitivity to parthanatos.
Hamano S, Noguchi T, Asai Y, Ito R, Komatsu R, Sato T, Inoue A, Maruyama T, Kudo TA, Hirata Y, Shindo S, Uchida Y, Hwang GW, Matsuzawa A. Hamano S, et al. Cell Death Discov. 2024 Feb 12;10(1):74. doi: 10.1038/s41420-024-01838-2. Cell Death Discov. 2024. PMID: 38346947 Free PMC article. - Genetic ablation of Nrf2/antioxidant response pathway in Alexander disease mice reduces hippocampal gliosis but does not impact survival.
Hagemann TL, Jobe EM, Messing A. Hagemann TL, et al. PLoS One. 2012;7(5):e37304. doi: 10.1371/journal.pone.0037304. Epub 2012 May 31. PLoS One. 2012. PMID: 22693571 Free PMC article.
References
- Gething MJ, Sambrook J: Protein folding in the cell. Nature 1992, 355:33-45 - PubMed
- Hartl FU: Molecular chaperones in cellular protein folding. Nature 1996, 381:571-580 - PubMed
- Hershko A, Ciechanover A: The ubiquitin system. Annu. Rev Biochem 1998, 67:425-479 - PubMed
- Grune T, Reinheckel T, Davies KJA: Degradation of oxidized proteins in mammalian cells. FASEB J 1997, 11:526-534 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous