Enhancement of presynaptic calcium current by cysteine string protein - PubMed (original) (raw)
Enhancement of presynaptic calcium current by cysteine string protein
Shan Chen et al. J Physiol. 2002.
Abstract
The isolated chick ciliary neuron calyx synapse preparation was used to test cysteine string protein (CSP) action on presynaptic N-type Ca(2+) channels. Endogenous CSP was localized primarily to secretory vesicle clusters in the presynaptic nerve terminal. Introduction of recombinant CSP into the voltage clamped terminal resulted in a prominent increase in Ca(2+) current amplitude. However, this increase could not be attributed to a change in Ca(2+) channel kinetics, voltage dependence, prepulse inactivation, or G protein inhibition but was attributed to the recruitment of dormant channels. Secretory vesicle associated endogenous CSP may play an important role in enhancing Ca(2+) channel activity at the transmitter release site.
Figures
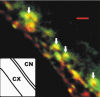
Figure 1. Image slice of a calyx nerve terminal (CX, inset cartoon) attached to a ciliary neuron (CN) and stained for CSP (green) or SV2 (red) with colocalized regions in yellow
Examples of transmitter release face synaptic vesicle clusters are indicated by arrows. Scale bar, 1 μm.
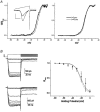
Figure 2. Enhancement of presynaptic calcium current by intracellular CSP
A, current traces from two calyces patched with pipettes containing either plain intracellular solution (inset lower right) or recombinant CSP (inset upper left) and recorded beginning immediately after seal rupture. The voltage protocol was a depolarizing step from −80 to 0 mV. Current amplitudes (I) were measured near the end of the step, normalized to that of the first trial (I_0, trace with dark line) and were plotted (with CSP, ○; control, •). B, currents activated by a depolarizing ramp protocol (top panel) recorded in a control calyx (middle panel) or with CSP (lower panel). Traces were recorded immediately after seal rupture (t = 0, bold) and at 20 (dotted) and 45 s (fine-dotted) thereafter. C, a series of ramp depolarizations were given as in Fig. 2_B at 5 s intervals to CSP-infused (□) and vector-control-infused (▪) calyx nerve terminals. The slope conductance, G, was determined as the slope of a straight line fitted to the ascending limb of the current peak between +35 and +40 mV and this was normalized to the value of G at t = 0 (_G_0). n = 4 for each treatment.
Figure 3. Effect of CSP on the time course of the Ca2+ current
Recordings are from three calyces (A-C) before (thick line) and after (thin line) perfusion through the patch electrode with CSP with voltage protocols above each panel. Raw (leak subtracted) data are shown in the left panel to compare current amplitudes while traces normalized to the current amplitude at the end of the step are on the right to compare current pulse shape. A and B are with a single pulse protocol depolarizing from −80 to 0 mV for 40 ms. In C the test pulse was preceded by a strong (+80 mV, 30 ms) prepulse to remove any voltage-sensitive inhibition.
Figure 4. Effect of intracellular CSP on calcium current inactivation
A, conductance-voltage relation. Current traces were recorded during a ramp depolarization protocol (inset, protocol as in Fig. 2_B_) before (thick line) and after (thin line) CSP-induced growth. Current traces were corrected for driving force using an _E_Ca of +65 mV and were normalized to _G_MAX (at ∼+30 mV) and were plotted against the membrane potential to yield _G/G_MAX-voltage curves. Curves before (thick line) and after (thin line) CSP action are shown for a single calyx (left panel) and pooled data (right panel). B, prepulse inactivation (PPI) curve. Ca2+ current PPI was determined using the voltage protocol in the upper left panel with an interval of 5 s between trials. Traces are shown for control (middle left panel) and CSP-facilitated (lower left panel) calyces. Pooled data normalized to current amplitude with a holding potential of −80 mV from four calyces and fitted Boltzmann relations are plotted before (•, continuous line) and after (○, dashed line) the CSP effect in the right panel.
Similar articles
- Presynaptic calcium channels and the depletion of synaptic cleft calcium ions.
Stanley EF. Stanley EF. J Neurophysiol. 2000 Jan;83(1):477-82. doi: 10.1152/jn.2000.83.1.477. J Neurophysiol. 2000. PMID: 10634889 - Presynaptic calcium-channel currents in normal and csp mutant Drosophila peptidergic terminals.
Morales M, Ferrús A, Martínez-Padrón M. Morales M, et al. Eur J Neurosci. 1999 May;11(5):1818-26. doi: 10.1046/j.1460-9568.1999.00604.x. Eur J Neurosci. 1999. PMID: 10215934 - Cysteine-string protein increases the calcium sensitivity of neurotransmitter exocytosis in Drosophila.
Dawson-Scully K, Bronk P, Atwood HL, Zinsmaier KE. Dawson-Scully K, et al. J Neurosci. 2000 Aug 15;20(16):6039-47. doi: 10.1523/JNEUROSCI.20-16-06039.2000. J Neurosci. 2000. PMID: 10934253 Free PMC article. - Interactions between proteins implicated in exocytosis and voltage-gated calcium channels.
Seagar M, Lévêque C, Charvin N, Marquèze B, Martin-Moutot N, Boudier JA, Boudier JL, Shoji-Kasai Y, Sato K, Takahashi M. Seagar M, et al. Philos Trans R Soc Lond B Biol Sci. 1999 Feb 28;354(1381):289-97. doi: 10.1098/rstb.1999.0380. Philos Trans R Soc Lond B Biol Sci. 1999. PMID: 10212477 Free PMC article. Review. - Cysteine strings, calcium channels and synaptic transmission.
Ganetzky B. Ganetzky B. Bioessays. 1994 Jul;16(7):461-3. doi: 10.1002/bies.950160704. Bioessays. 1994. PMID: 7945273 Review.
Cited by
- A syntaxin 1, Galpha(o), and N-type calcium channel complex at a presynaptic nerve terminal: analysis by quantitative immunocolocalization.
Li Q, Lau A, Morris TJ, Guo L, Fordyce CB, Stanley EF. Li Q, et al. J Neurosci. 2004 Apr 21;24(16):4070-81. doi: 10.1523/JNEUROSCI.0346-04.2004. J Neurosci. 2004. PMID: 15102922 Free PMC article. - Synaptic vesicles: test for a role in presynaptic calcium regulation.
Macleod GT, Marin L, Charlton MP, Atwood HL. Macleod GT, et al. J Neurosci. 2004 Mar 10;24(10):2496-505. doi: 10.1523/JNEUROSCI.5372-03.2004. J Neurosci. 2004. PMID: 15014125 Free PMC article. - Interaction between constitutively expressed heat shock protein, Hsc 70, and cysteine string protein is important for cortical granule exocytosis in Xenopus oocytes.
Smith GB, Umbach JA, Hirano A, Gundersen CB. Smith GB, et al. J Biol Chem. 2005 Sep 23;280(38):32669-75. doi: 10.1074/jbc.M501806200. Epub 2005 Jul 29. J Biol Chem. 2005. PMID: 16055447 Free PMC article. - Cysteine string protein limits expression of the large conductance, calcium-activated K⁺ (BK) channel.
Ahrendt E, Kyle B, Braun AP, Braun JE. Ahrendt E, et al. PLoS One. 2014 Jan 27;9(1):e86586. doi: 10.1371/journal.pone.0086586. eCollection 2014. PLoS One. 2014. PMID: 24475152 Free PMC article. - Neurons Export Extracellular Vesicles Enriched in Cysteine String Protein and Misfolded Protein Cargo.
Deng J, Koutras C, Donnelier J, Alshehri M, Fotouhi M, Girard M, Casha S, McPherson PS, Robbins SM, Braun JEA. Deng J, et al. Sci Rep. 2017 Apr 19;7(1):956. doi: 10.1038/s41598-017-01115-6. Sci Rep. 2017. PMID: 28424476 Free PMC article.
References
- Bezprozvanny I, Scheller RH, Tsien RW. Functional impact of syntaxin on gating of N-type and Q-type calcium channels. Nature. 1995;378:623–626. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous