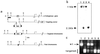A mutation in the peroxisome proliferator-activated receptor gamma-binding site in the gene for the cytosolic form of phosphoenolpyruvate carboxykinase reduces adipose tissue size and fat content in mice - PubMed (original) (raw)
A mutation in the peroxisome proliferator-activated receptor gamma-binding site in the gene for the cytosolic form of phosphoenolpyruvate carboxykinase reduces adipose tissue size and fat content in mice
Yael Olswang et al. Proc Natl Acad Sci U S A. 2002.
Abstract
Regulation of the turnover of triglycerides in adipose tissue requires the continuous provision of 3-glycerophosphate, which may be supplied by the metabolism of glucose or by glyceroneogenesis, the de novo synthesis of 3-glycerophosphate from sources other than hexoses or glycerol. The importance of glyceroneogenesis in adipose tissue was assessed in mice by specifically eliminating the expression of the cytosolic form of phosphoenolpyruvate carboxykinase (PEPCK-C), an enzyme that plays a pivotal role in the pathway. To accomplish this, we mutated the binding site for the peroxisome proliferator-activated receptor gamma (PPAR gamma) called the peroxisome proliferator-activated receptor element (PPARE), in the 5' flanking region of the PEPCK-C gene in the mouse by homologous recombination. The mutation abolished expression of the gene in white adipose tissue and considerably reduced its expression in brown adipose tissue, whereas the level of PEPCK-C mRNA in liver and kidney remained normal. Epididymal white adipose tissue from these mice had a reduced triglyceride deposition, with 25% of the animals displaying lipodystrophy. There was also a greatly reduced level of lipid accumulation in brown adipose tissue. A strong correlation between the hepatic content of triglycerides and the size of the epididymal fat pad in PPARE(-/-) mice suggests that hepatic triglyceride synthesis predominantly utilizes free fatty acids derived from the adipose tissue. Unlike other models, PPARE(-/-) mice with lipodystrophy did not exhibit the lipodystrophy-associated features of diabetes and displayed only moderate hyperglycemia. These studies establish the importance of the PPARE site for PEPCK-C gene expression in adipose tissue and the role of PEPCK-C in the regulation of glyceroneogenesis, a pathway critical for maintaining the deposition of triglycerides in adipose tissue.
Figures
Figure 1
Disruption of the PPARE element in the PEPCK-C gene. (a) The structures of the endogenous PEPCK gene, targeting vector, and the mutated chromosomes. The position of the PPARγ binding site in the 5′ flanking region of the gene is indicated (PP), and the horizontal arrow indicates the transcription start site (+1 bp). Restriction sites: E, _Eco_RI; H, _Hin_cII; X, _Xho_I; S, _Sma_I. Black triangles, loxP sites; neo, neomycin resistance gene; DT, diphtheria toxin-A chain gene. (b) Southern blot of _Eco_RI-digested genomic DNA from wild-type embryonic stem cells (lanes 1 and 3), targeted embryonic stem cells (lane 2), and homozygous mice (lane 4) hybridized to the probe indicated in a (as a thick line). The wild-type allele (11 kb) and targeted allele (2.5 kb) are indicated. (c) PCR products of genomic DNA using primers that span the PPARE site as specified in Methods. The products were digested with _Xho_I and separated by agarose gel electrophoresis. The wild-type allele (804 bp) and the doublet of the targeted allele (409 and 395 bp) are indicated.
Figure 2
The expression of PEPCK-C mRNA in wild-type and mutant mice. (a) Northern blot analysis of 15 and 30 μg total RNA from liver (L), kidney (K), and 10 or 20 μg (as indicated above) of brown adipose tissue (B) and white adipose tissue (A) PPARE+/+ (+/+), PPARE+/− (+/−), and PPARE−/− (−/−) mice. The blot was sequentially hybridized to a PEPCK-C cDNA probe, an 18S ribosomal RNA genomic fragment, and a UCP-1 cDNA probe and is representative of five independent experiments. (b) Reverse transcriptase–PCR was performed by using primers for PEPCK-C and β-actin as indicated in Methods. The RNA tested were from Liver (L), Kidney (K), and WAT (A) from PPARE−/− (−/−) and PPARE+/− (+/−) mice.
Figure 3
Effect of pyruvate on the release of FFA in vitro from adipose tissue. Two pieces (30–100 mg) from the distal thin part of epididymal fat pads from PPARE+/− (+/−; three mice) and PPARE−/− (−/−; four mice) mice after an 18-h fasting were incubated in vitro with (dotted bars) or without (hatched bars) 25 mM pyruvate. FFA levels (per gram of adipose tissue) in the incubation medium were measured after a 3-h incubation at 37°C.
Figure 4
The effect of the PPARE deletion on adipose tissue. Histological sections of WAT and BAT from PPARE+/− (+/−), PPARE−/− (−/−), and PPARE−/− with lipodystrophy (−/−*) were stained with hematoxylin and eosin.
Figure 5
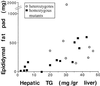
Correlation between liver triglyceride content and the weight of epididymal fat pads. Liver triglyceride content is plotted against the corresponding weights of both epididymal fat pads from the same mouse fasted for 18 h.
Similar articles
- Inhibition of glyceroneogenesis by histone deacetylase 3 contributes to lipodystrophy in mice with adipose tissue inflammation.
Zhang J, Henagan TM, Gao Z, Ye J. Zhang J, et al. Endocrinology. 2011 May;152(5):1829-38. doi: 10.1210/en.2010-0828. Epub 2011 Mar 15. Endocrinology. 2011. PMID: 21406501 Free PMC article. - PPAR gamma 2 regulates adipose expression of the phosphoenolpyruvate carboxykinase gene.
Tontonoz P, Hu E, Devine J, Beale EG, Spiegelman BM. Tontonoz P, et al. Mol Cell Biol. 1995 Jan;15(1):351-7. doi: 10.1128/MCB.15.1.351. Mol Cell Biol. 1995. PMID: 7799943 Free PMC article. - Phosphoenolpyruvate carboxykinase (Pck1) helps regulate the triglyceride/fatty acid cycle and development of insulin resistance in mice.
Millward CA, Desantis D, Hsieh CW, Heaney JD, Pisano S, Olswang Y, Reshef L, Beidelschies M, Puchowicz M, Croniger CM. Millward CA, et al. J Lipid Res. 2010 Jun;51(6):1452-63. doi: 10.1194/jlr.M005363. Epub 2010 Feb 2. J Lipid Res. 2010. PMID: 20124556 Free PMC article. - Glyceroneogenesis revisited.
Hanson RW, Reshef L. Hanson RW, et al. Biochimie. 2003 Dec;85(12):1199-205. doi: 10.1016/j.biochi.2003.10.022. Biochimie. 2003. PMID: 14739071 Review. - Cell-specific expression of cytosolic phosphoenolpyruvate carboxykinase in transgenic mice.
Beale EG, Clouthier DE, Hammer RE. Beale EG, et al. FASEB J. 1992 Dec;6(15):3330-7. doi: 10.1096/fasebj.6.15.1281456. FASEB J. 1992. PMID: 1281456 Review.
Cited by
- Function of phosphoenolpyruvate carboxykinase in mammary gland epithelial cells.
Hsieh CW, Huang C, Bederman I, Yang J, Beidelschies M, Hatzoglou M, Puchowicz M, Croniger CM. Hsieh CW, et al. J Lipid Res. 2011 Jul;52(7):1352-62. doi: 10.1194/jlr.M012666. Epub 2011 Apr 19. J Lipid Res. 2011. PMID: 21504969 Free PMC article. - Metformin can mitigate skeletal dysplasia caused by Pck2 deficiency.
Li Z, Yue M, Heng BC, Liu Y, Zhang P, Zhou Y. Li Z, et al. Int J Oral Sci. 2022 Nov 15;14(1):54. doi: 10.1038/s41368-022-00204-1. Int J Oral Sci. 2022. PMID: 36376276 Free PMC article. - Monoamine oxidase mediated oxidative stress: a potential molecular and biochemical crux in the pathogenesis of obesity.
Niveta JPS, John CM, Arockiasamy S. Niveta JPS, et al. Mol Biol Rep. 2023 Dec 23;51(1):29. doi: 10.1007/s11033-023-08938-9. Mol Biol Rep. 2023. PMID: 38142252 Review. - PEPCK-C reexpression in the liver counters neonatal hypoglycemia in Pck1 del/del mice, unmasking role in non-gluconeogenic tissues.
Semakova J, Hyroššová P, Méndez-Lucas A, Cutz E, Bermudez J, Burgess S, Alcántara S, Perales JC. Semakova J, et al. J Physiol Biochem. 2017 Feb;73(1):89-98. doi: 10.1007/s13105-016-0528-y. Epub 2016 Oct 26. J Physiol Biochem. 2017. PMID: 27785616 - Mitochondrial (dys)function in adipocyte (de)differentiation and systemic metabolic alterations.
De Pauw A, Tejerina S, Raes M, Keijer J, Arnould T. De Pauw A, et al. Am J Pathol. 2009 Sep;175(3):927-39. doi: 10.2353/ajpath.2009.081155. Epub 2009 Aug 21. Am J Pathol. 2009. PMID: 19700756 Free PMC article. Review.
References
- Vaughan M J. J Biol Chem. 1962;237:3354–3358. - PubMed
- Lin E C. Annu Rev Biochem. 1977;46:765–795. - PubMed
- Reshef L, Ballard F J, Hanson R W. J Biol Chem. 1970;245:5779–5785. - PubMed
- Ballard F J, Hanson R W, Leveille G A. J Biol Chem. 1967;242:2746–2750. - PubMed
- Reshef L, Niv J, Shapiro B. J Lipid Res. 1967;8:688–691. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases