Intracellular survival of Neisseria gonorrhoeae in male urethral epithelial cells: importance of a hexaacyl lipid A - PubMed (original) (raw)
Intracellular survival of Neisseria gonorrhoeae in male urethral epithelial cells: importance of a hexaacyl lipid A
Deborah M B Post et al. Infect Immun. 2002 Feb.
Abstract
Neisseria gonorrhoeae is a strict human pathogen that invades and colonizes the urogenital tracts of males and females. Lipooligosaccharide (LOS) has been shown to play a role in gonococcal pathogenesis. The acyl transferase MsbB is involved in the biosynthesis of the lipid A portion of the LOS. In order to determine the role of an intact lipid A structure on the pathogenesis of N. gonorrhoeae, the msbB gene was cloned and sequenced, a deletion and insertion mutation was introduced into N. gonorrhoeae, and the mutant strain was designated 1291A11K3. Mass spectrometric analyses of 1291A11K3 LOS determined that this mutation resulted in a pentaacyl rather than a hexaacyl lipid A structure. These analyses also demonstrated an increase in the phosphorylation of lipid A and an increase in length of the oligosaccharide of a minor species of the msbB LOS. The interactions of this mutant with male urethral epithelial cells (uec) were examined. Transmission and scanning electron microscopy studies indicated that the msbB mutants formed close associations with and were internalized by the uec at levels similar to those of the parent strain. Gentamicin survival assays performed with 1291A11K3 and 1291 bacteria demonstrated that there was no difference in the abilities of the two strains to adhere to uec; however, significantly fewer 1291A11K3 bacteria than parent strain bacteria were recovered from gentamicin-treated uec. These studies suggest that the lipid A modification in the N. gonorrhoeae msbB mutant may render it more susceptible to innate intracellular killing mechanisms when internalized by uec.
Figures
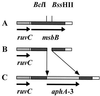
FIG. 1.
A deletion insertion mutant was made in the N. meningitidis msbB gene. (A) The N. meningitidis PCR product from the pNMBA11 plasmid was cloned into _Xba_I-_Hin_dIII-restricted pUC19. (B) A deletion was made in the msbB gene by restriction with _Bcl_I and _Bss_HII. (C) The pNMBA11K3 plasmid was generated by ligating the kanamycin resistance gene, _aphA_-3, into the sites of deletion of the msbB gene.
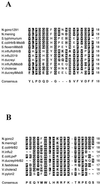
FIG. 2.
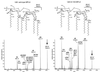
Homology analyses of predicted amino acid sequences of HtrB and MsbB from various bacteria. Residues with homology are shaded in black, and residues with similarity are shaded in grey. The consensus sequence is shown at the bottom of each panel. (A) Alignment of conserved region one (N. meningitidis strain NMB MsbB amino acids 186 to 203). (B) Alignment of conserved region two (N. meningitidis strain NMB MsbB amino acids 272 to 290). N.gono1291 and N.gono2, N. gonorrhoeae MsbB (GenBank accession no. AY057903); N.mening and N.mening2, N. meningitidis MsbB (GenBank accession no. AF428103); S. typhimurium, S. enterica serovar Typhimurium MsbB (GenBank accession no. AAD03801); E.coliHtrB-MsbB and E.coliHtrB2, E. coli strain K-12 HtrB and MsbB (SwissProt accession no. P24187 and P24205); S.flexerniMsbB, Shigella flexerni MsbB (GenBank accession no. AAB58154); H.influRdHtrB, H. influenzae strain Rd HtrB (GenBank accession no. AAC23173); H.influ2019 and H.influ2, H. influenzae strain 2019 HtrB (GenBank accession no. AAC43515); H.ducreyi and H.ducreyiHtrB2, Haemophilus ducreyi HtrB (GenBank accession no. AAF34642); H.influRdMsbB, H. influenzae strain Rd MsbB (GenBank accession no. AAC21868); V.cholerae and V.cholerae2, Vibrio cholerae strain N16961 lauroyl transferase (HtrB) (GenBank accession no. AAF93389); H.ducreyiMsbB, H. ducreyi MsbB (GenBank accession no. AAF33777); E.coliLpxP, E. coli strain K-12 LpxP (GenBank accession no. AAB66658); X.fastidiosa, Xylella fastidiosa lauroyl transferase (GenBank accession no. AAF82917); H.pylori2, Helicobacter pylori strain 26695 IbpB (GenBank accession no. AAD07343).
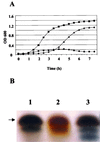
FIG.3.
Complementation of an E. coli htrB mutant with pNMBA11pUC19. (A) Growth curves, at 37°C, of MLK2 (▪), MLK217 (•), and MLK217A11 (▴). Data are representative of three separate experiments. (B) Silver-stained SDS-PAGE of LPS from MLK217A11 (lane 1), MLK217 (lane 2), and MLK2 (lane 3). Arrow, 4-kDa band.
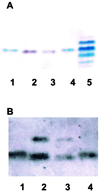
FIG. 4.
Characterization of 1291A11K3 LOS by SDS-PAGE analyses. (A) Silver-staining analysis of an SDS-PAGE gel. Lanes 1 and 4, 1291 LOS; lanes 2 and 3, 1291A11K3 LOS; lane 5, PID2 LOS. PID2 LOS is included as a molecular weight standard. (B) Western blot analysis using MAb 6B4. Lanes 1 and 4, 1291 LOS; lanes 2 and 3, 1291A11K3 LOS.
FIG. 5.
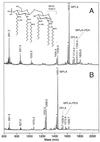
Negative-ion MALDI spectra of the _O_-deacylated LOS from N. gonorrhoeae strain 1291 (A) and N. gonorrhoeae strain 1291A11K3 (B). The major component of the 1291 _O_-deacylated LOS mixture. (M − H)− at m/z 2,792.9 contains a diphosphoryl lipid A and a nonasaccharide moiety bearing a single PEA group (see inset, top), consistent with the published structure of N. gonorrhoeae 1291 wild-type LOS (22). In the _O_-deacylated LOS mixture from the 1291A11K3 mutant, this species is also predominant but there is additional PEA heterogeneity in the sample. When present, the extra PEA group exists on the lipid A moiety (see inset, bottom), primarily on the reducing terminal phosphate (see text). Peaks marked with asterisks are (M − H-H2O)− species.
FIG. 6.
Negative-ion MALDI spectra of the lipid A fractions from N. gonorrhoeae strain 1291 (A) and N. gonorrhoeae strain 1291A11K3 (B). The major peaks in each spectrum correspond to monophosphoryl lipid A (MPLA) species. Under the acetic acid hydrolysis conditions used, the more labile reducing terminal phosphate of lipid A is partially removed (7). Minor amounts of diphosphoryl lipid A (DPLA) species and MPLA species bearing a PEA group are also present. The mass difference between the corresponding peaks in the two spectra is 182 Da, indicating that one of the two lauric acid residues present in the 1291 structure (inset) is missing in the 1291A11K3 lipid A. The peaks at m/z 1809.6 and 1626.7 in the spectra of the 1291 and 1291A11K3 lipid A molecules, respectively, are 53 Da above the corresponding MPLA+PEA peaks and are likely iron adducts, (M−3H+FeII)−. Peaks marked with asterisks are (M − H-H2O)− species.
FIG. 7.
Negative-ion electrospray MS/MS spectra of the monophosphorylated lipid A species from N. gonorrhoeae strain 1291 (left) and N. gonorrhoeae strain 1291A11K3 (right). The parent (M − H)− ions selected for collision-induced dissociation are indicated with arrows. Fragment ions are labeled on the spectra and indicated on the structures using a letter code. _O_-linked fatty acids are lost as free acids (a, b, c, and d cleavages) or ketenes (a', b', c', and d' cleavages). Fragments of type e and f are cross-ring cleavages.
FIG. 8.
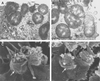
TEM of uec infected for 4 h with 1291A11K3 bacteria (A) or 1291 bacteria (B) (bar, 4 μm). SEM of uec infected for 4 h with 1291A11K3 bacteria (C) or 1291 bacteria (D) (bar, 300 nm). The thick arrows point to the uec plasma membrane, and the thin arrows point to the bacteria. The close association between the uec's plasma membrane and the bacteria is characteristic of clathrin-dependent receptor-mediated endocytosis.
FIG. 9.
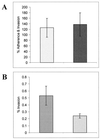
A representative example of the invasion assays performed on HPV-transduced uec using N. gonorrhoeae strains 1291 and 1291A11K3. (A) Mean values of percent adherence and invasion of 1291 ( ) and 1291A11K3 (
) and 1291A11K3 ( ) (P = 0.6947). (B) Mean values of percent invasion of 1291 (
) (P = 0.6947). (B) Mean values of percent invasion of 1291 ( ) and 1291A11K3 (
) and 1291A11K3 ( ) (P = 0.0130). Data are representative of six separate experiments. Adherence and invasion data were measured as the numbers of bacteria recovered from infected uec not treated with gentamicin. The invasion data were measured as the numbers of bacteria recovered from infected uec treated with gentamicin.
) (P = 0.0130). Data are representative of six separate experiments. Adherence and invasion data were measured as the numbers of bacteria recovered from infected uec not treated with gentamicin. The invasion data were measured as the numbers of bacteria recovered from infected uec treated with gentamicin.
Similar articles
- Lipooligosaccharide biosynthesis in pathogenic Neisseria. Cloning, identification, and characterization of the phosphoglucomutase gene.
Zhou D, Stephens DS, Gibson BW, Engstrom JJ, McAllister CF, Lee FK, Apicella MA. Zhou D, et al. J Biol Chem. 1994 Apr 15;269(15):11162-9. J Biol Chem. 1994. PMID: 8157643 - Membrane glycerophospholipid biosynthesis in Neisseria meningitidis and Neisseria gonorrhoeae: identification, characterization, and mutagenesis of a lysophosphatidic acid acyltransferase.
Swartley JS, Balthazar JT, Coleman J, Shafer WM, Stephens DS. Swartley JS, et al. Mol Microbiol. 1995 Nov;18(3):401-12. doi: 10.1111/j.1365-2958.1995.mmi_18030401.x. Mol Microbiol. 1995. PMID: 8748025 - Hexa-acylated lipid A is required for host inflammatory response to Neisseria gonorrhoeae in experimental gonorrhea.
Zhou X, Gao X, Broglie PM, Kebaier C, Anderson JE, Thom N, Apicella MA, Sempowski GD, Duncan JA. Zhou X, et al. Infect Immun. 2014 Jan;82(1):184-92. doi: 10.1128/IAI.00890-13. Epub 2013 Oct 14. Infect Immun. 2014. PMID: 24126526 Free PMC article. - The lytic transglycosylases of Neisseria gonorrhoeae.
Chan YA, Hackett KT, Dillard JP. Chan YA, et al. Microb Drug Resist. 2012 Jun;18(3):271-9. doi: 10.1089/mdr.2012.0001. Epub 2012 Mar 20. Microb Drug Resist. 2012. PMID: 22432703 Free PMC article. Review. - Defenses against oxidative stress in Neisseria gonorrhoeae: a system tailored for a challenging environment.
Seib KL, Wu HJ, Kidd SP, Apicella MA, Jennings MP, McEwan AG. Seib KL, et al. Microbiol Mol Biol Rev. 2006 Jun;70(2):344-61. doi: 10.1128/MMBR.00044-05. Microbiol Mol Biol Rev. 2006. PMID: 16760307 Free PMC article. Review.
Cited by
- The use of resazurin as a novel antimicrobial agent against Francisella tularensis.
Schmitt DM, O'Dee DM, Cowan BN, Birch JW, Mazzella LK, Nau GJ, Horzempa J. Schmitt DM, et al. Front Cell Infect Microbiol. 2013 Dec 6;3:93. doi: 10.3389/fcimb.2013.00093. eCollection 2013. Front Cell Infect Microbiol. 2013. PMID: 24367766 Free PMC article. - Gonococcal cervicitis: a role for biofilm in pathogenesis.
Steichen CT, Shao JQ, Ketterer MR, Apicella MA. Steichen CT, et al. J Infect Dis. 2008 Dec 15;198(12):1856-61. doi: 10.1086/593336. J Infect Dis. 2008. PMID: 18973432 Free PMC article. - Lipid A heterogeneity and its role in the host interactions with pathogenic and commensal bacteria.
Saha S, Pupo E, Zariri A, van der Ley P. Saha S, et al. Microlife. 2022 Jun 10;3:uqac011. doi: 10.1093/femsml/uqac011. eCollection 2022. Microlife. 2022. PMID: 37223360 Free PMC article. Review. - UVliPiD: A UVPD-Based Hierarchical Approach for De Novo Characterization of Lipid A Structures.
Morrison LJ, Parker WR, Holden DD, Henderson JC, Boll JM, Trent MS, Brodbelt JS. Morrison LJ, et al. Anal Chem. 2016 Feb 2;88(3):1812-20. doi: 10.1021/acs.analchem.5b04098. Epub 2016 Jan 15. Anal Chem. 2016. PMID: 26728944 Free PMC article. - Two lytic transglycosylases in Neisseria gonorrhoeae impart resistance to killing by lysozyme and human neutrophils.
Ragland SA, Schaub RE, Hackett KT, Dillard JP, Criss AK. Ragland SA, et al. Cell Microbiol. 2017 Mar;19(3):10.1111/cmi.12662. doi: 10.1111/cmi.12662. Epub 2016 Nov 3. Cell Microbiol. 2017. PMID: 27597434 Free PMC article.
References
- Apicella, M. A. 1974. Antigenically distinct population of Neisseria gonorrhoeae: isolation and characterization of the responsible determinants. J. Infect. Dis. 130:619-625. - PubMed
- Apicella, M. A., M. R. Ketterer, F. K. N. Lee, D. Zhou, P. A. Rice, and M. S. Blake. 1996. The pathogenesis of gonococcal urethritis in men: confocal and immunoelectron microscopic analysis of urethral exudates from men infected with Neisseria gonorrhoeae. J. Infect. Dis. 173:636-646. - PubMed
- Apicella, M. A., R. E. Mandrell, M. Shero, M. E. Wilson, J. McLeod Griffiss, G. F. Brooks, C. Lammel, J. F. Breen, and P. A. Rice. 1990. Modification of sialic acid of Neisseria gonorrhoeae lipooligosaccharide epitope expression in human urethral exudates: an immunoelectron microscopic analysis. J. Infect. Dis. 162:506-512. - PubMed
- Ausubel, F. M., R. Brent, R. E. Kingston, D. D. Moore, J. G. Seidman, J. A. Smith, and K. Struhl (ed.). 1987. Current protocols in molecular biology, vol. 1. John Wiley & Sons, New York, N.Y.
- Boue, S. M., and R. B. Cole. 2000. Confirmation of the structure of lipid A from Enterobacter agglomerans by electrospray ionization tandem mass spectrometry. J. Mass Spectrom. 35:361-368. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- AI45424/AI/NIAID NIH HHS/United States
- AI31254/AI/NIAID NIH HHS/United States
- R01 AI045728/AI/NIAID NIH HHS/United States
- RR01614/RR/NCRR NIH HHS/United States
- DK25295/DK/NIDDK NIH HHS/United States
- AI44642/AI/NIAID NIH HHS/United States
- T32AI07511/AI/NIAID NIH HHS/United States
- R01 AI031254/AI/NIAID NIH HHS/United States
- P01 AI044642/AI/NIAID NIH HHS/United States
- P41 RR001614/RR/NCRR NIH HHS/United States
- AI45778/AI/NIAID NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases