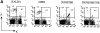Autoreactive B cells in the marginal zone that express dual receptors - PubMed (original) (raw)
Autoreactive B cells in the marginal zone that express dual receptors
Yijin Li et al. J Exp Med. 2002.
Abstract
Allotype and isotype exclusion is a property of most lymphocytes. The reason for this property is not known but it guarantees a high concentration of a single receptor, and threshold numbers of receptors may be required for efficient positive and negative selection. Receptor editing compromises exclusion by sustaining recombination even after a functional receptor is formed. Consequently, B cells expressing multiple receptors arise. We have studied such B cells in which one of the two receptors is anti-self, and find that these partially autoreactive B cells accumulate in the marginal zone. The restriction of these cells in this location may help to prevent them from undergoing diversification and developing into fully autoreactive B cells.
Figures
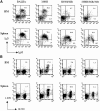
Figure 1.
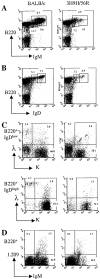
FACS® analysis of mature B cells in anti-DNA H chain sd-tg mice. (A) Splenic and bone marrow cells were isolated from 3H9H, 3H9H/56R, and 3H9H/56R/76R sd-tg mice. Cells were stained with antibodies against B220, CD43, IgM, and IgD. All analyses were performed on the lymphocyte-gated population. IgM+IgD+ cells were plotted from the B220+CD43−-gated population. (B) Recognition of transgenic H chain–bearing cells by the anti-idiotypic Ab, 1.209, which recognizes the 3H9 H chain in combination with most L-chain. Cells were double-stained with anti-B220 and 1.209. Data were plotted and percentages calculated from the lymphocyte-gated population. These results are representative of four independent experiments.
Figure 2.
κ and λ expression in spleen cells of mice with H chain of different DNA binding affinities. (A) Spleen cells from BALB/c, 3H9, 3H9H/56R, 3H9H/56R/76R sd-tg mice were stained with anti-κ and -λ. Cells were gated on a lymphoid gate, and percentages of κ+, λ+, and κ/λ+ are indicated. (B) 3H9H/56R mice have a large population of κ/λ double-positive B cells. Spleen cells from 3H9H/56R and the nontransgenic littermate were stained with anti-B220, κ, and λ. Dead cells were excluded by propidium iodide staining. Percentages of κ+, λ+, and κ/λ+ cells in a B220+ gate are indicated. Representative of experiments using three different mice of each kind. (C) Surface IgM expression of κ/λ double-positive B cells are higher than most of the κ single-positive B cells in 3H9H/56R mice. Histograms of IgM expression are shown for the κ/λ double-positive (R4 and thin line) and κ single-positive (R5 and bold line) population.
Figure 2.
κ and λ expression in spleen cells of mice with H chain of different DNA binding affinities. (A) Spleen cells from BALB/c, 3H9, 3H9H/56R, 3H9H/56R/76R sd-tg mice were stained with anti-κ and -λ. Cells were gated on a lymphoid gate, and percentages of κ+, λ+, and κ/λ+ are indicated. (B) 3H9H/56R mice have a large population of κ/λ double-positive B cells. Spleen cells from 3H9H/56R and the nontransgenic littermate were stained with anti-B220, κ, and λ. Dead cells were excluded by propidium iodide staining. Percentages of κ+, λ+, and κ/λ+ cells in a B220+ gate are indicated. Representative of experiments using three different mice of each kind. (C) Surface IgM expression of κ/λ double-positive B cells are higher than most of the κ single-positive B cells in 3H9H/56R mice. Histograms of IgM expression are shown for the κ/λ double-positive (R4 and thin line) and κ single-positive (R5 and bold line) population.
Figure 2.
κ and λ expression in spleen cells of mice with H chain of different DNA binding affinities. (A) Spleen cells from BALB/c, 3H9, 3H9H/56R, 3H9H/56R/76R sd-tg mice were stained with anti-κ and -λ. Cells were gated on a lymphoid gate, and percentages of κ+, λ+, and κ/λ+ are indicated. (B) 3H9H/56R mice have a large population of κ/λ double-positive B cells. Spleen cells from 3H9H/56R and the nontransgenic littermate were stained with anti-B220, κ, and λ. Dead cells were excluded by propidium iodide staining. Percentages of κ+, λ+, and κ/λ+ cells in a B220+ gate are indicated. Representative of experiments using three different mice of each kind. (C) Surface IgM expression of κ/λ double-positive B cells are higher than most of the κ single-positive B cells in 3H9H/56R mice. Histograms of IgM expression are shown for the κ/λ double-positive (R4 and thin line) and κ single-positive (R5 and bold line) population.
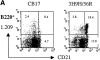
Figure 3.
Increase of MZ B cells in the H chain transgenic mice. (A) Spleen cells from BALB/c, 3H9, 3H9H/56R, 3H9H/56R/76R sd-tg mice were stained with anti-B220, -CD21, and -CD23. Percentages of CD21high and CD23low populations in the B220+ gate are indicated. (B) Increase of MZ B cells in the H chain sd-tg mice shown by immunohistochemistry. Spleen sections of each mouse were stained with anti-IgM that stains both MZ and follicular B cell (blue) and MOMA-1 that detects metallophilic marginal macrophage (red).
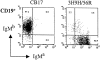
Figure 4.
The κ/λ double-positive B cells in 3H9H/56R are MZ B cells. Histograms of CD23, CD21, and IgD expression are shown for the κ/λ double-positive (R4 and thin line) and κ single-positive (R5 and bold line) population. Representative of experiments using three different mice.
Figure 5.
Allelic exclusion by the 3H9H/56R H chain transgene. Spleen cells from 3H9H/56R mice and the nontransgenic littermate were stained with anti-CD19, -IgMa, and -IgMb. Percentages of IgMa+ (the transgenic allele) and IgMb+ (the endogenous allele) cells in the CD19+ gate are indicated. Representative of two independent experiments using a different mouse of each kind.
Figure 6.
Detection of Id-positive B cells in 3H9H/56R mice. (A) The majority of Id-positive B cells in 3H9H/56R mice are in the MZ. Spleen cells from 3H9H/56R mice and the nontransgenic littermate were stained with anti-B220, -CD21, and 1.209 anti-Id antibodies. Percentages of CD21high and Id-positive cells in the B220+ gate are indicated. Representative of experiments using three different mice of each kind. (B) The κ/λ double-positive B cells in 3H9H/56R are Id positive. Histogram of 1.209 anti-Id staining are shown for the κ/λ double-positive (R4 and thin line) and κ single-positive (R5 and bold line) populations. Representative of experiments using three different mice.
Figure 6.
Detection of Id-positive B cells in 3H9H/56R mice. (A) The majority of Id-positive B cells in 3H9H/56R mice are in the MZ. Spleen cells from 3H9H/56R mice and the nontransgenic littermate were stained with anti-B220, -CD21, and 1.209 anti-Id antibodies. Percentages of CD21high and Id-positive cells in the B220+ gate are indicated. Representative of experiments using three different mice of each kind. (B) The κ/λ double-positive B cells in 3H9H/56R are Id positive. Histogram of 1.209 anti-Id staining are shown for the κ/λ double-positive (R4 and thin line) and κ single-positive (R5 and bold line) populations. Representative of experiments using three different mice.
Figure 7.
Loss of immature and mature/recirculating B cells and absence of κ/λ and Id-positive B cells in the bone marrow of 3H9H/56R mice. (A and B) Bone marrow cells from 3H9H/56R mice and the nontransgenic littermate were stained with anti-B220, -IgM, and -IgD. Percentages of B cells in the subsets of IgMlowB220low, IgMhighB220low, IgM+B220high, IgDlowB220low, and IgDhighB220high are indicated. (C) Bone marrow cells gated from IgDlowB220low and IgDhighB220high are shown for their light chain expression. Percentages of κ+, λ+, and κ/λ+ cells are indicated. (D) Bone marrow cells are stained with anti-B220, -IgM, and 1.209. Percentages of Id+, IgM+, and Id/IgM+ cells in a B220+ gate are indicated.
Figure 8.
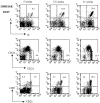
Increase of κ/λ double-positive, MZ, and Id-positive B cells in the spleen of 3H9H/56R mice with age. Spleen cells from 3H9H/56R mice of age 5, 7.5, and 11 wk were stained with anti-B220, -κ, -λ, -CD21, -CD23, and 1.209 anti-Id antibodies. Percentages of κ/λ double-positive B cell, CD21high and CD23low MZ B cells, and Id-positive cells in the B220+ gate are indicated.
Similar articles
- Allelic and isotypic light chain inclusion in peripheral B cells from anti-DNA antibody transgenic C57BL/6 and BALB/c mice.
Witsch EJ, Bettelheim E. Witsch EJ, et al. J Immunol. 2008 Mar 15;180(6):3708-18. doi: 10.4049/jimmunol.180.6.3708. J Immunol. 2008. PMID: 18322176 - Anti-DNA B cells in MRL/lpr mice show altered differentiation and editing pattern.
Li Y, Li H, Ni D, Weigert M. Li Y, et al. J Exp Med. 2002 Dec 16;196(12):1543-52. doi: 10.1084/jem.20021560. J Exp Med. 2002. PMID: 12486097 Free PMC article. - B cell deletion, anergy, and receptor editing in "knock in" mice targeted with a germline-encoded or somatically mutated anti-DNA heavy chain.
Pewzner-Jung Y, Friedmann D, Sonoda E, Jung S, Rajewsky K, Eilat D. Pewzner-Jung Y, et al. J Immunol. 1998 Nov 1;161(9):4634-45. J Immunol. 1998. PMID: 9794392 - Tolerance-induced receptor selection: scope, sensitivity, locus specificity, and relationship to lymphocyte-positive selection.
Aït-Azzouzene D, Skog P, Retter M, Kouskoff V, Hertz M, Lang J, Kench J, Chumley M, Melamed D, Sudaria J, Gavin A, Martensson A, Verkoczy L, Duong B, Vela J, Nemazee D, Alfonso C. Aït-Azzouzene D, et al. Immunol Rev. 2004 Feb;197:219-30. doi: 10.1111/j.0105-2896.2004.0106.x. Immunol Rev. 2004. PMID: 14962198 Review. - B-cell antigen receptor competence regulates B-lymphocyte selection and survival.
Buhl AM, Nemazee D, Cambier JC, Rickert R, Hertz M. Buhl AM, et al. Immunol Rev. 2000 Aug;176:141-53. doi: 10.1034/j.1600-065x.2000.00613.x. Immunol Rev. 2000. PMID: 11043774 Review.
Cited by
- Forced usage of positively charged amino acids in immunoglobulin CDR-H3 impairs B cell development and antibody production.
Ippolito GC, Schelonka RL, Zemlin M, Ivanov II, Kobayashi R, Zemlin C, Gartland GL, Nitschke L, Pelkonen J, Fujihashi K, Rajewsky K, Schroeder HW Jr. Ippolito GC, et al. J Exp Med. 2006 Jun 12;203(6):1567-78. doi: 10.1084/jem.20052217. Epub 2006 Jun 5. J Exp Med. 2006. PMID: 16754718 Free PMC article. - Light chain editing generates polyreactive antibodies in chronic graft-versus-host reaction.
Witsch EJ, Cao H, Fukuyama H, Weigert M. Witsch EJ, et al. J Exp Med. 2006 Jul 10;203(7):1761-72. doi: 10.1084/jem.20060075. Epub 2006 Jun 26. J Exp Med. 2006. PMID: 16801398 Free PMC article. - Germinal center exclusion of autoreactive B cells is defective in human systemic lupus erythematosus.
Cappione A 3rd, Anolik JH, Pugh-Bernard A, Barnard J, Dutcher P, Silverman G, Sanz I. Cappione A 3rd, et al. J Clin Invest. 2005 Nov;115(11):3205-16. doi: 10.1172/JCI24179. Epub 2005 Oct 6. J Clin Invest. 2005. PMID: 16211091 Free PMC article. - Targeting Toll-like receptor signaling in plasmacytoid dendritic cells and autoreactive B cells as a therapy for lupus.
Lenert PS. Lenert PS. Arthritis Res Ther. 2006;8(1):203. doi: 10.1186/ar1888. Epub 2006 Jan 10. Arthritis Res Ther. 2006. PMID: 16542467 Free PMC article. Review. - Allelic exclusion of immunoglobulin genes: models and mechanisms.
Vettermann C, Schlissel MS. Vettermann C, et al. Immunol Rev. 2010 Sep;237(1):22-42. doi: 10.1111/j.1600-065X.2010.00935.x. Immunol Rev. 2010. PMID: 20727027 Free PMC article. Review.
References
- Alt, F.W., V. Enea, A.L. Bothwell, and D. Baltimore. 1980. Activity of multiple light chain genes in murine myeloma cells producing a single, functional light chain. Cell. 21:1–12. - PubMed
- Coleclough, C., R.P. Perry, K. Karjalainen, and M. Weigert. 1981. Aberrant rearrangements contribute significantly to the allelic exclusion of immunoglobulin gene expression. Nature. 290:372–378. - PubMed
- Yamagami, T., E. ten Boekel, C. Schaniel, J. Andersson, A. Rolink, and F. Melchers. 1999. Four of five RAG-expressing JCkappa−/− small pre-BII cells have no L chain gene rearrangements: detection by high-efficiency single cell PCR. Immunity. 11:309–316. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases