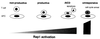Rap1 functions as a key regulator of T-cell and antigen-presenting cell interactions and modulates T-cell responses - PubMed (original) (raw)
Rap1 functions as a key regulator of T-cell and antigen-presenting cell interactions and modulates T-cell responses
Koko Katagiri et al. Mol Cell Biol. 2002 Feb.
Free PMC article
Abstract
Activation of T cells by antigen requires adhesive interactions with antigen-presenting cells (APC) in which leukocyte function-associated antigen 1 (LFA-1) and intercellular adhesion molecules (ICAMs) are important. However, it is not well understood what signaling molecules regulate this process and how the modulation of adhesive events influences T-cell activation. Here we show that Rap1 is activated in T cells in an antigen-dependent manner and accumulated at the contact site of T-cell and antigen-loaded APC. Inhibition of Rap1 activation by a dominant-negative Rap1 or SPA-1, a Rap1 GTPase-activating protein, abrogates LFA-1-ICAM-1-mediated adhesive interactions with antigen-pulsed APC and the subsequent T-cell-receptor triggering and interleukin-2 production. Conversely, augmented antigen-dependent Rap1 activation by the expression of wild-type Rap1 enhances these responses but culminates in apoptosis by Fas and FasL. Thus, Rap1 functions as a key regulator of T-cell and APC interactions and modulates T-cell responses from productive activation to activation-induced cell death by regulating the strength of adhesive interactions. Moreover, constitutive Rap1 activation rendered T cells unresponsive with accumulation of p27(Kip1). Our study indicates that the activation state of Rap1 has a decisive effect on the T-cell response to antigen.
Figures
FIG. 1.
TCR-induced adhesion of Jurkat cells to ICAM-1 is inhibited by Rap1N17 or SPA-1. (Aa) Adhesion of Jurkat cells transfected with vector alone, Rap1N17, and H-rasN17. Jurkat cells were stimulated with or without 1 μg of OKT3 (TCR) or 10 ng of PMA per ml for 30 min at 37°C in ICAM-1-coated plates, as described in Materials and Methods. The level of adhesion to BSA was <1%. Average and standard errors of triplicate experiments are shown. (Ab) IL-2 production upon stimulation with TCR-CD28 antibody cross-linking. Rap1N17 or pcDNA3 (vector) stable transfectants were stimulated by cross-linking of the TCR complex with OKT3 (TCR) or OKT3 and CD28.2 (TCR+CD28) for 16 h, and the supernatants were harvested for IL-2 measurement. An optical density at 437 nm of 1 was equal to 0.15 ng of recombinant mouse IL-2/ml. (Ba) The upper panel shows the induction of SPA-1 detected with anti-flag antibody. SPA-1 was uninduced (−) or induced (+) by infection with adenovirus expressing cre recombinase for 2 days. The lower panel shows the inhibition of TCR-mediated Rap1 activation by the induction of SPA-1 expression. Jurkat cells uninduced (−) or induced (+) to express SPA-1 were stimulated with OKT3 (TCR) for 10 min and lysed, and GTP-bound Rap1, H-ras, and Rac were detected with pull-down assays by using immobilized GST fusion proteins of RalGDS-RBD, Raf-RBD, and PAK-CD. Western blots of total cell lysates are also shown. (Bb) Adhesion of Jurkat cells uninduced (−) or induced (+) to express SPA-1 with OKT3 (TCR) or PMA. Jurkat cells transfected with Spa-1 or empty vector were infected with adenovirus carrying cre recombinase. Infected cells were stimulated with or without 1 μg of OKT3 (TCR) or 10 ng of PMA per ml for 30 min at 37°C in ICAM-1-coated plates, as described in Materials and Methods. Average and standard errors of triplicate experiments are shown. (Ca) Decrease in TCR-dependent Rap1 activation by costimulation with antibody cross-linking of CD28. Jurkat cells were stimulated with OKT3 in the absence (TCR) or presence of CD28.2 (TCR/CD28) for 10 min and lysed, and GTP-bound Rap1, H-ras, and Rac were measured as described in Fig. 1Ba. (Cb) TCR-induced adhesion of Jurkat cells was reduced by costimulation with CD28. Jurkat cells were unstimulated or stimulated with OKT3 (TCR), CD28.2 (CD28), or TS2/18 (CD2) alone or in combination for 30 min at 37°C in ICAM-1-coated plates. Average and standard errors of triplicate experiments are shown.
FIG. 2.
Cellular aggregation of T cells and APC via LFA-1-ICAM-1 accompanies activation and accumulation of Rap1 at the contact site. (Aa) The formation of large T-cell-APC clusters was dependent on LFA-1. HEL-specific _I-Ak_-restricted 3A9 T-cell hybridoma and _I-Ak_-bearing CH27 B cells were incubated in the absence (−) or presence of HEL (100 μg/ml) for 16 h with or without 20μg of anti-LFA-1 antibody/ml. Original magnification, ca. ×100. (Ab) LFA-1-ICAM-1-dependent IL-2 production. 3A9 T cells and CH27 B cells were cultured as described above with HEL and antibodies as indicated, and the supernatants were harvested for IL-2 measurement. An optical density at 437 nm of 1 was equal to 0.2 ng of recombinant mouse IL-2/ml. (B) Antigen-dependent activation of Rap1 in T cells. 3A9 T cells expressing T7-tagged WT-Rap1 were cultured with CH27 B cells without (−HEL) or with (+HEL) antigen for 4 and 8 h. GTP-bound Rap1 was analyzed as in Fig. 1B with pull-down of GST-RalGDS-RBD. Bound Rap1 (upper panels) and total Rap1 (lower panels) were detected by Western blotting with anti-T7 antibody. (C) Redistribution of Rap1 at the contact site. 3A9 T cells expressing WT-Rap1-GFP fusion protein or GFP protein were mixed with antigen-pulsed CH27 B cells for 30 min at 37°C. Conjugates were bound to poly-
l
-lysine-coated coverslips, fixed for 5 min with 3% formaldehyde, and observed under a confocal microscope (LSM510; Zeiss). Three representative confocal images of conjugates for WT-Rap1-GFP are shown. The left panels are Nomarski views of the same conjugates shown for GFP in the right panels. (D) Quantification of redistributed Rap1-GFP. The conjugates of WT-Rap1-GFP or GFP-expressing T cells with APC in panel C were imaged in 0.3-μm steps through the entire cell volume. The data shown represent the integral of the fluorescence intensity of the manually defined contact site or noncontact site over the total value of the cell. The manually defined contact or noncontact sites represent 10% of the cell volume. The relative enrichment (the fluorescence per unit volume at the contact site divided by the fluorescence per unit volume of the entire cell) of Rap1-GFP or of GFP is 2.01 ± 0.59 or 0.98 ± 0.12, respectively. Error bars represent the standard deviation.
FIG. 3.
Inhibition of LFA-1-ICAM-1-dependent T-cell-APC interaction and IL-2 production by Rap1N17 or SPA-1. (A) Inhibition of cell aggregation by Rap1N17 and SPA-1. 3A9 T cells were transfected with empty vector, or genes encoding Rap1N17, Spa-1, or H-rasN17, and more than five stable clones for each construct were isolated for the experiment. The result is representative of five independent clones, which gave similar results. They were cultured with CH27 B cells in the presence of antigen, as described in Fig. 2A. Original magnification, ×100. (B) Conjugate formation of 3A9 T cells (green) and CH27 B cells as APC (red) loaded with (+HEL) or without (No antigen) antigen. T cells were mixed with an equal number of APC and incubated for 30 min at 37°C. Nonspecific aggregates were disrupted by vortexing, and the samples were analyzed by flow cytometry. A representative set of two-dimensional plots is shown. The number in each plot is the percentage of conjugates. (C) Inhibition of IL-2 production by Rap1N17 and SPA-1. 3A9 T cells transfected with the vector alone, Rap1N17, or Spa-1 were incubated with CH27 B cells as described in Fig. 2A, and the supernatants were harvested for IL-2 measurement. An optical density at 437 nm of 1 was equal to 0.25 ng of recombinant mouse IL-2/ml. Bars represent the average and standard error of two representative experiments performed in triplicate with each of five clones.
FIG. 4.
Inhibition of conjugate formation of the OVA-specific T-cell clone and APC (A) and IL-2 production by SPA-1 (B). (A) Conjugate formation of T-cell clone (green) transfected with the neomycin gene (Neo) or Spa-1 and A20.2J B cells as APC (red) loaded with (+OVA) or without (No antigen) 1 mg of OVA per ml was analyzed as in Fig. 3B. The number in each plot is the percentage of conjugates. (B) IL-2 production. T-cell clones transfected with the neomycin gene (Neo) or Spa-1 were incubated with A20.2J B cells in the absence (None) or presence of antigen for 16 h with or without anti-LFA-1 or anti-ICAM-1 antibodies. T-cell clones were also stimulated with anti-CD3ɛ (2C11) and CD28 (37.51) for 16 h (TCR/CD28). The supernatants were harvested, and IL-2 concentrations were measured. An optical density at 437 nm of 1 was equal to 0.13 ng of recombinant mouse IL-2/ml. The average with the standard error of two representative experiments in triplicate is shown.
FIG. 5.
Enhanced T-cell-APC interaction by overexpression of WT-Rap1 promoted IL-2 production and TCR downregulation. (A) The upper panel shows the increased expression levels of Rap1 in WT-Rap1 transfectants. The 3A9 T cells transfected with vector alone and the representative clones expressing a modest level (WT-Rap1-m) and a high level (WT-Rap1-h) of Rap1 are shown. The levels of Rap1 expression were detected by Western blotting with monoclonal anti-Rap1 antibody. The lower panel shows the enhanced antigen-dependent activation of Rap1 in transfectants. The transfectants were cultured with CH27 B cells without (−HEL) or with antigen (+HEL) for 8 h. GTP-bound Rap1 was analyzed as in Fig. 1B with pull-down of GST-Ra1GDS-RBD. Bound Rap1 was detected by Western blotting with anti-Rap1 antibody. The relative levels of Rap1 quantitated by an LAS1000 apparatus (Fuji) are shown under each blot. (B) Expression of WT-Rap1 enhanced conjugate formation between 3A9 T cells and CH27 B cells in the presence of antigen. Transfectants were incubated with nonpulsed (No antigen) or antigen-pulsed (+HEL) CH27 B cells as APC and analyzed for conjugate formation as described in Fig. 3B. A representative set of two-dimensional plots of T cells (green) versus APC (red) is shown. The number in each plot is the percentage of conjugates. (C) Promotion of IL-2 production by expression of WT-Rap1. 3A9 T cells transfected with vector alone, as well as WT-Rap1-expressing cells, were incubated with APC for 8 h as described in Fig. 2A, and the supernatants were harvested for IL-2 measurement. An optical density at 437 nm of 1 was equal to 0.35 ng of recombinant mouse IL-2/ml. The average and the standard error of two representative experiments performed in triplicate with three WT-Rap1-expressing clones are shown. (D) Increased TCR downregulation after antigen stimulation in WT-Rap1 transfectants. 3A9 T cells transfected with vector alone or WT-Rap1 were stimulated with antigen-pulsed CH27 B cells in the presence (▪ and ▴) or absence (□ and ▵) of anti-LFA-1 antibody. SPA-1-expressing 3A9 T cells were also stimulated with antigen-pulsed CH27 B cells (○). TCR expression levels were assessed 1 and 3 h later by flow cytometry as described in Materials and Methods.
FIG. 6.
Enhancing T-cell-APC interaction resulted in activation-induced cell death. (A) Apoptosis of WT-Rap1-expressing 3A9T cells. (Aa) WT-Rap1-expressing 3A9 T cells (▩) were cultured with CH27 B cells (□) loaded with (HEL) or without (−) 100 μg HEL per ml for 16 h and then stained with PI and analyzed by flow cytometry. Anti-LFA-1 antibody was included as indicated. CH27 B cells were labeled with CFSE before culture to distinguish them from 3A9 T cells. The average and standard error of the percent PI positive for each population of three experiments are shown. (Ab) 3A9 T cells transfected with vector alone or with WT-Rap1 were cultured with CH27 B cells in the absence (−) or presence (+) of HEL antigen for 16 h. Anti-LFA-1 antibody (Ab) was included as indicated. Chromosomal DNA was extracted and analyzed by a 1.5% agarose electrophoresis to detect DNA fragmentation. (B) Apoptosis mediated by Fas/FasL. (Ba) WT-Rap1-expressing 3A9 T cells were cultured with CH27 B cells with (+) or without (−) HEL in the absence (−) or presence of soluble Fas-Fc (sFas) and TNFR1-Fc (sTNFR) chimeric proteins. DNA fragmentation was detected as described in Fig. 6Ab. (Bb) Upregulation of FasL expression in 3A9 T cells. 3A9T cells transfected with vector alone or with WT-Rap1 were cultured with APC in the presence or absence (−) of antigen. Anti-LFA-1 antibody was included as indicated. Cells harvested after 8 h were stained with anti-FasL antibody, and FITC-labeled anti-mouse immunoglobulin and then analyzed by flow cytometry. To gate out the APC, CH27 B cells were labeled with PKH-26 before culture. The data are shown for 3A9 T cells. CH27 B cells did not express FasL. The percentage of positive 3A9 T cells stained with the secondary antibody alone in each condition was <1%. The average and standard errors of two experiments are shown.
FIG. 7.
Suppression of IL-2 production and accumulation of p27Kip1 by Rap1V12. (A) Level of Rap1-GTP in Rap1V12- and WT-Rap1-expressing cells. 3A9 T cells expressing T7-tagged Rap1V12 and WT-Rap1 were cultured with CH27 B cells without (−HEL) or with antigen (+HEL) for 8 h. GTP-bound Rap1 was analyzed as in Fig. 1B with pull-down of GST-RalGDS-RBD. Bound Rap1 (upper) and total Rap1 (lower) were detected by Western blotting with anti-T7 antibody. (B) Rap1V12 increased LFA-1-ICAM-1-mediated adhesion of 3A9 T cells and accumulated at the contact site with APC in the absence of antigen. (Ba) Adhesion of 3A9 T cells transfected with vector alone, WT-Rap1, and Rap1V12. T cells were stimulated with or without 10 ng of PMA per ml for 30 min at 37°C in mouse ICAM-1-Ig-coated plates and analyzed as described in Materials and Methods. The average and standard errors of triplicate experiments are shown. (Bb) Cellular localization of Rap1V12. 3A9 T cells expressing T7-tagged Rap1V12 were mixed with CH27 B cells for 30 min at 37°C and immunostained with anti-T7 antibody and Alexa Fluor 488 goat anti-mouse IgG (Molecular Probes). Representative confocal images of Rap1V12 in T cells either unconjugated or conjugated with CH27 B cells are shown. The left panels are Nomarski views of the same cells shown for Rap1V12 in the right panels. Cells transfected with vector alone did not show any significant fluorescent signals under the same conditions. (C) Persistent and marked preactivation of Rap1 rendered T cells unresponsive to antigen. (Ca) Decreased IL-2 production in Rap1V12-expressing 3A9 T cells and OVA-specific T-cell clone. 3A9 T cells and the OVA-specific T-cell clone transfected with vector alone (Neo) or Rap1V12 were cultured with appropriate APC, as in Fig. 3 and 4, with (+) or without (−) antigen for 16 h. IL-2 concentrations of the supernatants were measured. An optical density at 437 nm of 1 was equal to 0.23 ng of recombinant mouse IL-2/ml. Bars represent the average and standard error of two representative experiments performed in triplicate. (Cb) Antigen-induced phosphorylation of ERKs in 3A9 T cells expressing Rap1V12 or WT-Rap1. 3A9 T cells transfected with the neomycin genes (Neo), WT-Rap1, and Rap1V12 were cultured for 1 h with CH27 B cells which were preloaded without (−HEL) or with antigen (+HEL) for 16 h and fixed with 1% paraformaldehyde. The phosphorylation levels of ERKs were examined by Western blotting with antiphosphorylated ERK1 (pERK1)- and ERK2 (pERK2)-specific antibodies (upper panel). The expression levels of total ERK1 and ERK2 are shown (lower panel). (D) Accumulation of p27Kip1 and cell cycle arrest in Rap1V12-expressing T cells. (Da) Increased expression levels of p27Kip1 in Rap1V12-expressing 3A9 T cells and OVA-specific T-cell clone were detected by Western blotting with anti-p27Kip1 antibody. (Db) Increased cell population in the G0/G1 phase caused by Rap1V12 expression. The OVA-specific T-cell clones transfected with vector alone (Neo) or Rap1V12 were stained with PI and analyzed by flow cytometry.
FIG. 8.
Spectrum of immunological responses modulated by Rap1. It can be seen that low levels of Rap1 activation do not induce T-cell-APC adhesive interactions via LFA-1-ICAM-1, resulting in a nonproductive T-cell response. Appropriate Rap1 activation does induce stable T-cell-APC association, leading to full T-cell activation. Overactivated Rap1 enhances T-cell-APC interactions and precipitates T cells into AICD. Extremely accumulated active Rap1 causes cell cycle arrest and renders T cells unresponsive to antigen stimulation.
Similar articles
- SLAT promotes TCR-mediated, Rap1-dependent LFA-1 activation and adhesion through interaction of its PH domain with Rap1.
Côte M, Fos C, Canonigo-Balancio AJ, Ley K, Bécart S, Altman A. Côte M, et al. J Cell Sci. 2015 Dec 1;128(23):4341-52. doi: 10.1242/jcs.172742. Epub 2015 Oct 19. J Cell Sci. 2015. PMID: 26483383 Free PMC article. - [Regulation of integrin-mediated adhesion and migration by Rap1].
Kinashi T. Kinashi T. Tanpakushitsu Kakusan Koso. 2002 Dec;47(16 Suppl):2200-5. Tanpakushitsu Kakusan Koso. 2002. PMID: 12518437 Review. Japanese. No abstract available. - Rap1 translates chemokine signals to integrin activation, cell polarization, and motility across vascular endothelium under flow.
Shimonaka M, Katagiri K, Nakayama T, Fujita N, Tsuruo T, Yoshie O, Kinashi T. Shimonaka M, et al. J Cell Biol. 2003 Apr 28;161(2):417-27. doi: 10.1083/jcb.200301133. Epub 2003 Apr 21. J Cell Biol. 2003. PMID: 12707305 Free PMC article. - Cytotoxic-T-lymphocyte antigen 4 receptor signaling for lymphocyte adhesion is mediated by C3G and Rap1.
Kloog Y, Mor A. Kloog Y, et al. Mol Cell Biol. 2014 Mar;34(6):978-88. doi: 10.1128/MCB.01024-13. Epub 2014 Jan 6. Mol Cell Biol. 2014. PMID: 24396067 Free PMC article. - T Cell Activation Pathways: B7, LFA-3, and ICAM-1 Shape Unique T Cell Profiles.
Wingren AG, Parra E, Varga M, Kalland T, Sjogren HO, Hedlund G, Dohlsten M. Wingren AG, et al. Crit Rev Immunol. 2017;37(2-6):463-481. doi: 10.1615/CritRevImmunol.v37.i2-6.130. Crit Rev Immunol. 2017. PMID: 29773030 Review.
Cited by
- Signal transduction pathways in chronic inflammatory autoimmune disease: small GTPases.
Reedquist KA, Tak PP. Reedquist KA, et al. Open Rheumatol J. 2012;6:259-72. doi: 10.2174/1874312901206010259. Epub 2012 Sep 7. Open Rheumatol J. 2012. PMID: 23028410 Free PMC article. - Rap1a null mice have altered myeloid cell functions suggesting distinct roles for the closely related Rap1a and 1b proteins.
Li Y, Yan J, De P, Chang HC, Yamauchi A, Christopherson KW 2nd, Paranavitana NC, Peng X, Kim C, Munugalavadla V, Kapur R, Chen H, Shou W, Stone JC, Kaplan MH, Dinauer MC, Durden DL, Quilliam LA. Li Y, et al. J Immunol. 2007 Dec 15;179(12):8322-31. doi: 10.4049/jimmunol.179.12.8322. J Immunol. 2007. PMID: 18056377 Free PMC article. - Dissecting the immunosuppressive tumor microenvironments in Glioblastoma-on-a-Chip for optimized PD-1 immunotherapy.
Cui X, Ma C, Vasudevaraja V, Serrano J, Tong J, Peng Y, Delorenzo M, Shen G, Frenster J, Morales RT, Qian W, Tsirigos A, Chi AS, Jain R, Kurz SC, Sulman EP, Placantonakis DG, Snuderl M, Chen W. Cui X, et al. Elife. 2020 Sep 10;9:e52253. doi: 10.7554/eLife.52253. Elife. 2020. PMID: 32909947 Free PMC article. - Unveiling the Innate and Adaptive Immunity Interplay: Global Transcriptomic Profiling of the Host Immune Response in Candida albicans Endophthalmitis in a Murine Model.
Khapuinamai A, Rudraprasad D, Pandey S, Mishra DK, Joseph J. Khapuinamai A, et al. ACS Omega. 2024 Sep 30;9(40):41491-41503. doi: 10.1021/acsomega.4c05081. eCollection 2024 Oct 8. ACS Omega. 2024. PMID: 39398165 Free PMC article. - PIPKI gamma 90 negatively regulates LFA-1-mediated adhesion and activation in antigen-induced CD4+ T cells.
Wernimont SA, Legate KR, Simonson WT, Fassler R, Huttenlocher A. Wernimont SA, et al. J Immunol. 2010 Oct 15;185(8):4714-23. doi: 10.4049/jimmunol.1001445. Epub 2010 Sep 20. J Immunol. 2010. PMID: 20855869 Free PMC article.
References
- Allen, P. M., and E. R. Unanue. 1984. Differential requirements for antigen processing by macrophages for lysozyme-specific T cell hybridomas. J. Immunol. 132:1077-1079. - PubMed
- Bachmann, M. F., K. Mckall-Faienza, R. Schmits, D. Bouchard, J. Beach, D. E. Speiser, T. W. Mak, and P. S. Ohashi. 1997. Distinct roles for LFA-1 and CD28 during activation of naive T cells: adhesion versus costimulation. Immunity 7:549-557. - PubMed
- Bachmann, M. F., A. Oxenius, D. E. Speiser, S. Mariathasan, H. Hengartner, R. M. Zinkernagel, and P. S. Ohashi. 1997. Peptide induced TCR-down regulation on naive T cell predicts agonist/partial agonist properties and strictly correlates with T cell activation. Eur. J. Immunol. 27:2195-2203. - PubMed
- Bianchi, E., S. Denti, A. Granata, G. Bossi, J. Geginat, A. Villa, L. Rogge, and R. Pardi. 2000. Integrin LFA-1 interacts with the transcriptional co-activator JAB1 to modulate Ap-1 activity. Nature 404:617-621. - PubMed
- Boussiotis, V. A., G. J. Freeman, A. Berezovskaya, D. L. Barber, and L. M. Nadler. 1997. Maintenance of human T cell anergy: blocking of IL-2 gene transcription by activated Rap1. Science 278:124-128. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous