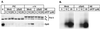Spt5 cooperates with human immunodeficiency virus type 1 Tat by preventing premature RNA release at terminator sequences - PubMed (original) (raw)
Spt5 cooperates with human immunodeficiency virus type 1 Tat by preventing premature RNA release at terminator sequences
Cyril F Bourgeois et al. Mol Cell Biol. 2002 Feb.
Abstract
The human immunodeficiency virus type 1 (HIV-1) Tat protein activates transcription elongation by stimulating the Tat-activated kinase (TAK/p-TEFb), a protein kinase composed of CDK9 and its cyclin partner, cyclin T1. CDK9 is able to hyperphosphorylate the carboxyl-terminal domain (CTD) of the large subunit of RNA polymerase during elongation. In addition to TAK, the transcription elongation factor Spt5 is required for the efficient activation of transcriptional elongation by Tat. To study the role of Spt5 in HIV transcription in more detail, we have developed a three-stage Tat-dependent transcription assay that permits the isolation of active preinitiation complexes, early-stage elongation complexes, and Tat-activated elongation complexes. Spt5 is recruited in the transcription complex shortly after initiation. After recruitment of Tat during elongation through the transactivation response element RNA, CDK9 is activated and induces hyperphosphorylation of Spt5 in parallel to the hyperphosphorylation of the CTD of RNA polymerase II. However, immunodepletion experiments demonstrate that Spt5 is not required for Tat-dependent activation of the kinase. Chase experiments using the Spt5-depleted extracts demonstrate that Spt5 is not required for early elongation. However, Spt5 plays an important role in late elongation by preventing the premature dissociation of RNA from the transcription complex at terminator sequences and reducing the amount of polymerase pausing at arrest sites, including bent DNA sequences. This novel biochemical function of Spt5 is analogous to the function of NusG, an elongation factor found in Escherichia coli that enhances RNA polymerase stability on templates and shows sequence similarity to Spt5.
Figures
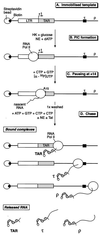
FIG. 1.
Three-stage Tat-dependent transcription reactions. (A) Immobilized template. The DNA template, containing the HIV LTR and an artificial terminator (τ), was biotinylated on both ends using Klenow DNA polymerase and bound to streptavidin-coated magnetic beads. (B) PIC formation. PIC are obtained following incubation of immobilized templates with a nuclear extract (NE) depleted of ATP by a treatment with HK and glucose. Phosphorylation of the CTD of RNA polymerase is allowed by adding dATP in the reaction mixture. Protein composition of preinitiation complexes can be analyzed by Western blotting. (C) Pausing at −14. Early elongation complexes were formed by incubation of the preinitiation complexes in the absence of ATP and in the presence of CTP, GTP, and [α-32P]UTP. This allows the RNA polymerase to carry out the transcription of the first 14 nt of the RNA chain and to pause before the first A residue located at +15 on the DNA template. (D) Chase. Elongation complexes were obtained by allowing the +14 complexes to extend following addition of all the nucleotides in the presence or absence of Tat. The population of elongation complexes, which have reached various points on the template, can be purified by washing the beads and analyzed for their RNA and/or protein composition. Released transcripts can be analyzed by purifying the RNA present in the supernatant collected after magnetic separation. The major stop sites correspond to the end of TAR, the terminator (τ), and the end of the template (ρ).
FIG. 2.
Efficient elongation from transcription complexes paused at +14 requires the addition of nuclear extract. (A) Preinitiation complexes were set up on immobilized templates as described in the legend to Fig. 1 and paused at +14 by elongation in the presence of CTP, GTP, and [α-32P]UTP. The +14 products were then washed and chased by addition of unlabeled nucleotides. Reactions were performed in the absence (−) or presence (+) of 10 μl of fresh nuclear extract (NE) and of 20 ng of Tat protein. RNA products tightly associated with the elongation complexes (bound) or prematurely released from the template (free) were analyzed: Abbreviations: TAR, paused transcription complexes or transcripts released at +59, at the end of the TAR stem-loop structure; Stem II, complexes released at +104, at the end of stem-loop II in the HIV sequence; τ, paused transcription complexes or transcripts released at the terminator; ρ, transcripts reaching the end of the template. (B) Transcription in the presence of NF-κB. Recombinant NFκB p65 protein (300 ng) purified from baculoviruses (+) or the corresponding storage buffer (NDBZ buffer; see Materials and Methods) (−) was added prior to the nuclear extract to set up the preinitiation complex. Transcription was then carried out to pause the complexes at+14, and beads were washed and then chased in the presence of fresh nuclear extract and the absence (−) or presence (+) of 20 ng of Tat. Bound or released (free) RNA products were analyzed.
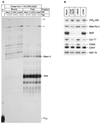
FIG. 3.
Spt5 prevents release of transcripts at terminator sequences. (A) Standard transcription reactions using an immobilized wild-type HIV-1 LTR template with either a mock-depleted (Mock) or Spt5-depleted (ΔSpt5) nuclear extract (NE), in the absence (−) or presence (+) of 20 ng of Tat. RNA products present in elongation complexes (bound fraction) as well as released transcripts (free fraction) were analyzed. (B) Densitometry of the gels shown in panel A. Continuous line, mock-depleted extracts in the presence of Tat; broken line, Spt5-depleted extracts in the presence of Tat.
FIG. 4.
Spt5-enhanced readthrough of transcription arrest sites. Three different templates were used in this experiment: the wild-type HIV-1 LTR (pW1), which contains the artificial stem-loop terminator sequence; constructs in which the terminator sequence has been replaced by an arrest sequence present in the histone H3.3 gene (H3.3); and a control sequence (control). Standard transcription reactions using the three different immobilized templates with either a mock-depleted (Mock) or a Spt5-depleted (ΔSpt5) nuclear extract (NE), in the absence (−) or presence (+) of 20 ng of Tat, were performed. RNA products present in elongation complexes (bound fraction) as well as released transcripts (free fraction) are shown.
FIG. 5.
Complementary activities of Spt5 and TAK during elongation. (A) Elongation complexes arrested at +14 were prepared using a nuclear extract depleted of both TAK and Spt5. The +14 complexes were washed and subsequently chased in the presence of either mock-depleted (Mock), TAK-depleted (ΔTAK), or Spt5-depleted (ΔSpt5) nuclear extracts (NE). Each chase was performed in the absence (−) or presence (+) of 20 ng of Tat protein. RNA products present in elongation complexes (bound fraction) as well as released transcripts (free fraction) were analyzed. (B) Immunoblot showing the protein composition of depleted nuclear extracts. A corresponding amount of each nuclear extract was analyzed by immunoblotting using antibodies directed against the following proteins (from top to bottom): TAFII250, the core of RNA polymerase II (Pol II) (N-20), Spt5 (anti-DSIF-p160), cyclin T1, CDK9, CDK7, and RAP74.
FIG. 6.
Spt5 is recruited shortly after initiation and hyperphosphorylated during elongation in the presence of Tat. (A) Immunoblot showing the protein composition of elongation complexes. Standard transcription reactions were performed using templates carrying either wild-type (WT), bulge (mGC), or loop (mLG) mutants of the TAR element, in the presence of 100 ng of LacR and in the absence (−) or presence (+) of 20 ng of Tat. Elongation complexes were purified and analyzed with antibodies directed (from top to bottom) against the core of RNA polymerase II (N-20), Spt5 (provided by R. Gaynor) or LacR (NL1.1B2.10). Tat induces hyperphosphorylation of both RNA polymerase (IIo*) and Spt5 (Spt5*). A 1-μl volume of nuclear extract (E) was used as a control. (B) Phosphatase treatment of elongation complexes. Elongation complexes purified as in panel A were incubated in the absence (−) or presence (+) of protein phosphatase 1 (PP1) and analyzed by immunoblotting with Spt5 antibodies. (C) Immunoblot showing the protein composition of the different transcription complexes. PIC and +14 complexes were assembled on wild-type immobilized template and purified as described in the legend to Fig. 1. Elongation complexes were purified from parallel standard transcription reactions, using templates carrying wild-type or mutant (mGC) TAR. Tat was added (+) or not (−) at different times to monitor its effect in different complexes. Purified complexes were loaded on precast gels of appropriate concentration for analysis of different proteins and probed with the corresponding antibodies (from top to bottom): Spt5 (provided by R. Gaynor), the core of RNA polymerase II (N-20), CDK9 (H-169), cyclin T1 (C-20), CDK7 (C-19), and cyclin H (D-10).
FIG. 7.
Spt5 is not required for Tat-dependent hyperphosphorylation of RNA polymerase II. (A) Elongation complexes were assembled using mock-depleted extracts (Mock) or immunodepleted extracts with anti-CDK7 antibodies (ΔCDK7) or a combination of antibodies against CDK9 and cyclin T1 (ΔTAK). The complexes were analyzed by Western blotting using antibodies directed against (from top to bottom) Spt5 (provided by R. Gaynor), the core of RNA polymerase II (N-20), CDK7 (C-19), CDK9 (H-169), and cyclin T1 (C-20). (B) Elongation complexes were assembled using mock-depleted extracts or extracts immunodepleted of Spt5 and analyzed with antibodies directed against (from top to bottom) the core of RNA polymerase II (N-20), Spt5 and CDK9 (H-169). NE, nuclear extract.
FIG. 8.
Spt5 recruitment is independent of RNA polymerase II phosphorylation. (A) PIC and early elongation complexes (+14) were assembled as described in the legend to Fig. 1, using mock-depleted (Mock) or Spt5-depleted (ΔSpt5) nuclear extract (NE) pretreated with HK and glucose, and in the presence of increasing levels of dATP (5, 10, or 25 μM). The protein compositions of the different transcription complexes were analyzed by Western blotting with core RNA polymerase II (N-20)- or Spt5 (provided by R. Gaynor)-specific antibodies. (B) Transcription gel showing the corresponding +14 RNA products. Note that RNA polymerase can be extensively phosphorylated under conditions that do not permit formation of the +14 products. Spt5 is recruited proportionally to the amount of the +14 product.
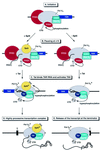
FIG. 9.
Model for the activity of Spt5 in the Tat-mediated transactivation of transcription at the HIV-1 LTR promoter. (A) Initiation. The RNA polymerase II (Pol II) complex, containing all the general transcription factors assembles on the promoter (only TFIID and TFIIH are represented). CDK9 and cyclin T1 are also present in the complex but are inactive (blue boxes). During initiation, the CTD of RNA polymerase is extensively phosphorylated by the CDK7 subunit of TFIIH. (B) Pausing at +14. Shortly after initiation, as measured by the +14 complexes, Spt5 is recruited to the transcription complex. Assembly of the +14 complex and phosphorylation of the CTD by TFIIH are equivalent in the presence and absence of Spt5. (C) Tat binds TAR RNA and activates TAK. Shortly after promoter clearance, the general transcription factors, including TFIIH, dissociate from the transcription complex. After the transcription of the TAR element, Tat is recruited and activates TAK (the CDK9 and CycT1 complex represented by blue circles). The active kinase complex phosphorylates both the CTD of RNA polymerase II (II0* form) and Spt5 (Spt5*). (D) Highly processive transcription complex. In the presence of Spt5, the highly processive transcription complex formed after Tat-mediated activation is stabilized and can carry out the transcription of the full HIV genome. (E) Release of the transcript at the terminator. In the absence of Spt5, the transcription complex containing a highly phosphorylated RNA polymerase II is less stable than complexes containing Spt5. As a result, there is an increased rate of release of transcripts at pause sites, such as the terminator sequence (τ).
Similar articles
- Phosphorylation of the RNA polymerase II carboxyl-terminal domain by CDK9 is directly responsible for human immunodeficiency virus type 1 Tat-activated transcriptional elongation.
Kim YK, Bourgeois CF, Isel C, Churcher MJ, Karn J. Kim YK, et al. Mol Cell Biol. 2002 Jul;22(13):4622-37. doi: 10.1128/MCB.22.13.4622-4637.2002. Mol Cell Biol. 2002. PMID: 12052871 Free PMC article. - Tat modifies the activity of CDK9 to phosphorylate serine 5 of the RNA polymerase II carboxyl-terminal domain during human immunodeficiency virus type 1 transcription.
Zhou M, Halanski MA, Radonovich MF, Kashanchi F, Peng J, Price DH, Brady JN. Zhou M, et al. Mol Cell Biol. 2000 Jul;20(14):5077-86. doi: 10.1128/MCB.20.14.5077-5086.2000. Mol Cell Biol. 2000. PMID: 10866664 Free PMC article. - Regulatory functions of Cdk9 and of cyclin T1 in HIV tat transactivation pathway gene expression.
Romano G, Kasten M, De Falco G, Micheli P, Khalili K, Giordano A. Romano G, et al. J Cell Biochem. 1999 Dec 1;75(3):357-68. J Cell Biochem. 1999. PMID: 10536359 Review. - Tackling Tat.
Karn J. Karn J. J Mol Biol. 1999 Oct 22;293(2):235-54. doi: 10.1006/jmbi.1999.3060. J Mol Biol. 1999. PMID: 10550206 Review.
Cited by
- The cell biology of HIV-1 latency and rebound.
Mbonye U, Karn J. Mbonye U, et al. Retrovirology. 2024 Apr 5;21(1):6. doi: 10.1186/s12977-024-00639-w. Retrovirology. 2024. PMID: 38580979 Free PMC article. Review. - The PNUTS-PP1 complex acts as an intrinsic barrier to herpesvirus KSHV gene expression and replication.
Devlin AM, Shukla A, Ruiz JC, Barnes SD, Govindan A, Hunter OV, Scarborough AM, D'Orso I, Conrad NK. Devlin AM, et al. Nat Commun. 2022 Dec 2;13(1):7447. doi: 10.1038/s41467-022-35268-4. Nat Commun. 2022. PMID: 36460671 Free PMC article. - The pleiotropic roles of SPT5 in transcription.
Song A, Chen FX. Song A, et al. Transcription. 2022 Feb-Jun;13(1-3):53-69. doi: 10.1080/21541264.2022.2103366. Epub 2022 Jul 25. Transcription. 2022. PMID: 35876486 Free PMC article. Review. - So Pathogenic or So What?-A Brief Overview of SIV Pathogenesis with an Emphasis on Cure Research.
Kleinman AJ, Pandrea I, Apetrei C. Kleinman AJ, et al. Viruses. 2022 Jan 12;14(1):135. doi: 10.3390/v14010135. Viruses. 2022. PMID: 35062339 Free PMC article. Review. - Induction of Autophagy to Achieve a Human Immunodeficiency Virus Type 1 Cure.
Campbell GR, Spector SA. Campbell GR, et al. Cells. 2021 Jul 16;10(7):1798. doi: 10.3390/cells10071798. Cells. 2021. PMID: 34359967 Free PMC article. Review.
References
- Awrey, D. E., R. G. Weilbaecher, S. A. Hemming, S. M. Orlicky, C. M. Kane, and A. M. Edwards. 1997. Transcription elongation through DNA arrest sites: a multistep process involving both RNA polymerase II subunit RPB9 and TFIIS. J. Biol. Chem. 272:14747-14754. - PubMed
- Berkhout, B., R. H. Silverman, and K.-T. Jeang. 1989. Tat trans-activates the human immunodeficiency virus through a nascent RNA target. Cell 59:273-282. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous