Wnt/beta-catenin/Tcf signaling induces the transcription of Axin2, a negative regulator of the signaling pathway - PubMed (original) (raw)
Wnt/beta-catenin/Tcf signaling induces the transcription of Axin2, a negative regulator of the signaling pathway
Eek-hoon Jho et al. Mol Cell Biol. 2002 Feb.
Abstract
Axin2/Conductin/Axil and its ortholog Axin are negative regulators of the Wnt signaling pathway, which promote the phosphorylation and degradation of beta-catenin. While Axin is expressed ubiquitously, Axin2 mRNA was seen in a restricted pattern during mouse embryogenesis and organogenesis. Because many sites of Axin2 expression overlapped with those of several Wnt genes, we tested whether Axin2 was induced by Wnt signaling. Endogenous Axin2 mRNA and protein expression could be rapidly induced by activation of the Wnt pathway, and Axin2 reporter constructs, containing a 5.6-kb DNA fragment including the promoter and first intron, were also induced. This genomic region contains eight Tcf/LEF consensus binding sites, five of which are located within longer, highly conserved noncoding sequences. The mutation or deletion of these Tcf/LEF sites greatly diminished induction by beta-catenin, and mutation of the Tcf/LEF site T2 abolished protein binding in an electrophoretic mobility shift assay. These results strongly suggest that Axin2 is a direct target of the Wnt pathway, mediated through Tcf/LEF factors. The 5.6-kb genomic sequence was sufficient to direct the tissue-specific expression of d2EGFP in transgenic embryos, consistent with a role for the Tcf/LEF sites and surrounding conserved sequences in the in vivo expression pattern of Axin2. Our results suggest that Axin2 participates in a negative feedback loop, which could serve to limit the duration or intensity of a Wnt-initiated signal.
Figures
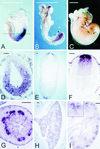
FIG.1.
Expression of Axin2 mRNA during mouse embryogenesis. (A to C) Whole-mount in situ hybridization. At E7.5 (A), Axin2 is expressed throughout the posterior region of the embryo; at E8.5 (B), expression is seen in the rostral and caudal ends of the neural ectoderm. At E9.5 (data not shown) and E10.5 (C), expression is seen along the entire length of the dorsal neural tube, as well as in the branchial arches (arrowheads) and limb buds (arrows). (D to F) In situ hybridization to transverse sections. At E7.5 (D), Axin2 is expressed in both the primitive streak mesoderm (arrow) and the posterior embryonic ectoderm (arrowhead). The approximate orientation of panel D is shown by the dotted line in panel A. At E10.5 (E) and E11.5 (F), strong expression is seen in the roof plate of the neural tube, with progressively weaker expression in a stripe of cells extending ventrally. The approximate orientation of panel E is shown by the dotted line in panel C. (G to I) Sections of developing organs from E14.5 embryos. In the lung (G), expression is seen throughout epithelial component. In the gut (H), Axin2 is expressed specifically in the epithelium at the base of the nascent villi, where the crypts will later form. In the kidney (I), expression is seen specifically in the ureteric buds; inset, higher magnification of branching ureteric bud tip. Bars, 1 mm (A to C) and 0.1 mm (D to I).
FIG. 2.
Induction of Axin2 mRNA by Wnts in cell culture and ex vivo tissue culture systems. (A) Induction of Axin2 mRNA in Wnt1/5-expressing C57MG cells. Total RNA was isolated from C57MG cells stably transfected with either lacZ or Wnt1/5 expression vectors, and RT-PCR was performed to measure the level of Axin2, Axin, and β-catenin mRNAs. (B and C) Induction of Axin2 mRNA and protein in LiCl-treated 293T cells. 293T cells were treated with 40 mM LiCl for different times, and RT-PCR and Western blot (WB) analysis were performed to measure the level of Axin2 mRNA and protein as well as control β-actin and GSK-3β. (D) Induction of Axin2 mRNA in embryonic gut endoderm cocultured with Wnt1-expressing NIH 3T3 cells. One-millimeter pieces of rat embryonic endoderm from E13 embryos were cocultured on either _lacZ_- or Wnt1-expressing NIH 3T3 cells for 24 h. Total RNA was isolated, and RT-PCR was performed with primer pairs specific for rat Axin2, as well as rat cytokeratin 19 (which cross-reacts with mouse cytokeratin 19) and GAPDH as controls.
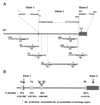
FIG. 3.
Axin2 promoter and intron 1 sequences contain potential Tcf/LEF binding sites within longer conserved DNA sequences. (A) A 5.6-kb DNA fragment containing the Axin2 promoter, exon 1, intron 1, and part of exon 2 was subcloned from a mouse BAC clone and sequenced. Exons are shown as gray boxes, and the sequence is numbered with bp 1 corresponding to the start of the cDNA sequence. Eight conserved Tcf/LEF binding sites (underlined and designated T1 to T8) were identified. The site-directed mutations introduced into each site (Fig. 5) are shown on top of the original sequence. (B) Conservation of five potential Tcf/LEF binding sites and surrounding sequences between human and mouse Axin2 promoter/intron 1 sequences. Sequence comparison between mouse and human BAC clones revealed strong conservation of sites T2, T3, T4, T5, and T8, as well as between 106 and 151 bp of the surrounding DNA sequence. The conserved sequences are as follows: T2, −128 to −3; T3, 262 to 412; T4 and T5, 701 to 806; and T8, 2534 to 2646.
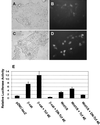
FIG. 4.
The Axin2 promoter and intron 1 direct Wnt- or β-catenin-inducible expression of reporter genes. (A) Expression of d2EGFP under the Axin2 promoter/intron 1 is induced by β-catenin. Axin2 promoter/intron 1 sequence (−2883 to +2703) was cloned into the pd2EGFP-1 vector (Ax2-d2EGFP). Ax2-d2EGFP was cotransfected into 293T cells together with either control LacZ (A and B) or a wild-type hemagglutinin-tagged β-catenin expression plasmid (C and D), and transient expression of d2EGFP was monitored. Fluorescence microscopy shows induction of d2EGFP expression by β-catenin (D) compared to the lacZ control (B). (E) Axin2/luciferase activity is induced by Wnt1/5 or β-catenin and inhibited by dominant-negative Tcf-4E. The Axin2 promoter/intron 1 sequence (−2883 to +2703) was cloned into the vector pGL3-Basic to produce Ax2-Luc. Ax2-Luc and pRL-TK (Renilla luciferase under the thymidine kinase [TK] promoter) were cotransfected into 293T cells together with the indicated plasmids, and the ratio of Ax2-driven fruit fly luciferase to TK-driven constitutively expressed Renilla luciferase activity was measured. Cotransfection of either β-catenin or Wnt1/5 increased the ratio, indicating induction. Addition of Tcf increased the degree of induction, while the addition of dominant-negative Tcf-4E (DN) blocked the induction by β-catenin or Wnt1/5. The error bars indicate standard deviations.
FIG. 5.
Mutation or deletion of Tcf/LEF binding sites in the Axin2 promoter/intron 1 leads to reduced induction by β-catenin. Axin2 promoter/intron 1 sequences were used to drive expression of luciferase reporter constructs. Vertical bars indicate exon 1 and part of exon 2. The lower six constructs shown lacked intron 1. Intact Tcf/LEF binding sites are indicated by ○, single nucleotide mutations (see Fig. 3A for specific mutations) are indicated by X, and a triple mutation in site T2 (from 5"-CTTT
GAT
-3" to 5"-CTTT
CGC
-3") is indicated by 3X. The fold induction of luciferase activity was measured after cotransfection with β-catenin and compared to the lacZ control, as described in the legend to Fig. 4. Error bars show standard deviations.
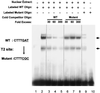
FIG. 6.
Mutation of a potential Tcf/LEF binding site abolishes shifted DNA-protein complexes in an EMSA. Duplex oligonucleotides containing wild-type (WT) or mutated T2 sites (from 5"-CTTT
GAT
-3" to 5"-CTTT
CGC
-3") were incubated with 293T cell nuclear extract (lanes 2 to 9), and DNA-protein complexes were separated in 5% native polyacrylamide gels. No shifted band was detected without nuclear extract (lanes 1 and 10). Mutation of site T2 caused a clear reduction in the shifted bands (compare lanes 2 and 9). An increasing amount of wild-type cold oligonucleotide efficiently eliminates the shifted bands while mutated oligonucleotide does not (compare lanes 3 to 5 with lanes 6 to 8).
FIG. 7.
Expression of Ax2/d2EGFP in transgenic embryos and developing organs is similar to the endogenous expression of Axin2. (A and C) Dark field; (E and G) bright field; (B, D, F, and H) d2EGFP fluorescence. (A and B) At E8.5, d2EGFP driven by the Axin2 promoter/intron 1 is strongly expressed in the head folds, tail bud region, and dorsal neural tube. (C and D) At E10.5, d2EGFP is expressed along the full length of the dorsal neural tube, as well as in the branchial arches and limb buds and in regions of the brain. Endogenous Axin2 is also expressed in the brain at a similar stage, although the sites have not been well characterized (Zhang and Costantini, unpublished). (E and F) Expression of d2EGFP in the ureteric bud tips of a transgenic E14.5 kidney (right) but not in a wild-type control kidney (left). (G and H) Expression of d2EGFP in E14.5 transgenic lung epithelium.
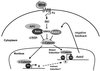
FIG. 8.
A model for the role of Axin2 in Wnt signal transduction. Upon transduction of a Wnt signal, transcription of the Axin2 gene is induced via the β-catenin/Tcf pathway. Our point mutation analysis, as well as data from cotransfection of DN-Tcf, suggests that Axin2 expression is controlled by Tcf/LEF factors. In addition to direct induction, Axin2 may be further induced by an indirect mechanism, in which Tcf enhances the expression of unknown transcription factor(s) X, which in turn enhances Axin2 expression through a mechanism not requiring the Tcf/LEF binding sites. Thus, Axin2 may provide a negative feedback loop for the down regulation of β-catenin to normal levels after a Wnt signal.
Similar articles
- Activation of AXIN2 expression by beta-catenin-T cell factor. A feedback repressor pathway regulating Wnt signaling.
Leung JY, Kolligs FT, Wu R, Zhai Y, Kuick R, Hanash S, Cho KR, Fearon ER. Leung JY, et al. J Biol Chem. 2002 Jun 14;277(24):21657-65. doi: 10.1074/jbc.M200139200. Epub 2002 Apr 8. J Biol Chem. 2002. PMID: 11940574 - Lef/Tcf-dependent Wnt/beta-catenin signaling during Xenopus axis specification.
Geng X, Xiao L, Lin GF, Hu R, Wang JH, Rupp RA, Ding X. Geng X, et al. FEBS Lett. 2003 Jul 17;547(1-3):1-6. doi: 10.1016/s0014-5793(03)00639-2. FEBS Lett. 2003. PMID: 12860376 - Regulation of lymphoid enhancer factor 1/T-cell factor by mitogen-activated protein kinase-related Nemo-like kinase-dependent phosphorylation in Wnt/beta-catenin signaling.
Ishitani T, Ninomiya-Tsuji J, Matsumoto K. Ishitani T, et al. Mol Cell Biol. 2003 Feb;23(4):1379-89. doi: 10.1128/MCB.23.4.1379-1389.2003. Mol Cell Biol. 2003. PMID: 12556497 Free PMC article. - Signaling through beta-catenin and Lef/Tcf.
Novak A, Dedhar S. Novak A, et al. Cell Mol Life Sci. 1999 Oct 30;56(5-6):523-37. doi: 10.1007/s000180050449. Cell Mol Life Sci. 1999. PMID: 11212302 Free PMC article. Review. - TCF: Lady Justice casting the final verdict on the outcome of Wnt signalling.
Brantjes H, Barker N, van Es J, Clevers H. Brantjes H, et al. Biol Chem. 2002 Feb;383(2):255-61. doi: 10.1515/BC.2002.027. Biol Chem. 2002. PMID: 11934263 Review.
Cited by
- Macrophages contribute to the cyclic activation of adult hair follicle stem cells.
Castellana D, Paus R, Perez-Moreno M. Castellana D, et al. PLoS Biol. 2014 Dec 23;12(12):e1002002. doi: 10.1371/journal.pbio.1002002. eCollection 2014 Dec. PLoS Biol. 2014. PMID: 25536657 Free PMC article. - Coordinated changes in the expression of Wnt pathway genes following human and rat peripheral nerve injury.
van Vliet AC, Lee J, van der Poel M, Mason MRJ, Noordermeer JN, Fradkin LG, Tannemaat MR, Malessy MJA, Verhaagen J, De Winter F. van Vliet AC, et al. PLoS One. 2021 Apr 13;16(4):e0249748. doi: 10.1371/journal.pone.0249748. eCollection 2021. PLoS One. 2021. PMID: 33848304 Free PMC article. - AML1-ETO mediates hematopoietic self-renewal and leukemogenesis through a COX/β-catenin signaling pathway.
Zhang Y, Wang J, Wheat J, Chen X, Jin S, Sadrzadeh H, Fathi AT, Peterson RT, Kung AL, Sweetser DA, Yeh JR. Zhang Y, et al. Blood. 2013 Jun 13;121(24):4906-16. doi: 10.1182/blood-2012-08-447763. Epub 2013 May 3. Blood. 2013. PMID: 23645839 Free PMC article. - Azelaic acid stimulates catalase activation and promotes hair growth through upregulation of Gli1 and Gli2 mRNA and Shh protein.
Amirfakhryan E, Davarnia B, Jeddi F, Najafzadeh N. Amirfakhryan E, et al. Avicenna J Phytomed. 2020 Sep-Oct;10(5):460-471. Avicenna J Phytomed. 2020. PMID: 32995324 Free PMC article. - Activation of Wnt/β-catenin signaling increases apoptosis in melanoma cells treated with trail.
Zimmerman ZF, Kulikauskas RM, Bomsztyk K, Moon RT, Chien AJ. Zimmerman ZF, et al. PLoS One. 2013 Jul 15;8(7):e69593. doi: 10.1371/journal.pone.0069593. Print 2013. PLoS One. 2013. PMID: 23869245 Free PMC article.
References
- Behrens, J., B. A. Jerchow, M. Wurtele, J. Grimm, C. Asbrand, R. Wirtz, M. Kuhl, D. Wedlich, and W. Birchmeier. 1998. Functional interaction of an axin homolog, conductin, with beta-catenin, APC, and GSK3beta. Science 280:596-599. - PubMed
- Bienz, M., and H. Clevers. 2000. Linking colorectal cancer to Wnt signaling. Cell 103:311-320. - PubMed
- Cadigan, K. M., and R. Nusse. 1997. Wnt signaling: a common theme in animal development. Genes Dev. 11:3286-3305. - PubMed
- Eastman, Q., and R. Grosschedl. 1999. Regulation of LEF-1/TCF transcription factors by Wnt and other signals. Curr. Opin. Cell Biol. 11:233-240. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous