Dynamin-dependent transferrin receptor recycling by endosome-derived clathrin-coated vesicles - PubMed (original) (raw)
Comparative Study
Dynamin-dependent transferrin receptor recycling by endosome-derived clathrin-coated vesicles
Ellen M van Dam et al. Mol Biol Cell. 2002 Jan.
Abstract
Previously we described clathrin-coated buds on tubular early endosomes that are distinct from those at the plasma membrane and the trans-Golgi network. Here we show that these clathrin-coated buds, like plasma membrane clathrin-coated pits, contain endogenous dynamin-2. To study the itinerary that is served by endosome-derived clathrin-coated vesicles, we used cells that overexpressed a temperature-sensitive mutant of dynamin-1 (dynamin-1(G273D)) or, as a control, dynamin-1 wild type. In dynamin-1(G273D)-expressing cells, 29-36% of endocytosed transferrin failed to recycle at the nonpermissive temperature and remained associated with tubular recycling endosomes. Sorting of endocytosed transferrin from fluid-phase endocytosed markers in early endosome antigen 1-labeled sorting endosomes was not inhibited. Dynamin-1(G273D) associated with accumulated clathrin-coated buds on extended tubular recycling endosomes. Brefeldin A interfered with the assembly of clathrin coats on endosomes and reduced the extent of transferrin recycling in control cells but did not further affect recycling by dynamin-1(G273D)-expressing cells. Together, these data indicate that the pathway from recycling endosomes to the plasma membrane is mediated, at least in part, by endosome-derived clathrin-coated vesicles in a dynamin-dependent manner.
Figures
Figure 1
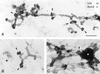
Clathrin-coated buds on endosomes contain endogenous dynamin. Nontransfected HeLa cells were incubated in the presence of Tf/HRP for 60 min at 37°C, and processed for whole mount immunoelectron microscopy. Samples were immuno-double–labeled for clathrin (cla, 10-nm gold) and endogenous dynamin-2 (dyn2, 5-nm gold) using polyclonal anti-clathrin and monoclonal HUDY-1, respectively. (A–C) Tubular early endosomes varied in length (compare A with B), but all were decorated with buds that labeled for both clathrin and dynamin-2 (for examples, see arrowheads). For quantification, see Figure 9A. (B) A large electron-lucent dynamin/clathrin-coated pit at the plasma membrane (arrow) is clearly different from DAB-contrasted 60-nm buds on endosomes (arrowheads). (C) Occasionally, tubules and buds (arrowhead) seem to associate with early endosomal vacuoles (asterisk). Bar, 200 nm.
Figure 2
Expression of dynwt and dynts and the effects on TfR internalization. (A) Cells were cultured for 6 d at 32°C in the presence (+) or absence (−) of tetracycline for the last 1–6 d. After extensive washing and replacement of tetracycline-containing medium by tetracycline-lacking medium, medium was either left on the cells for 6 d (− refresh medium) or refreshed daily (+ refresh medium). Western blots of cell lysates were probed with anti-HA antibodies to demonstrate HA-tagged dynwt or dynts and with anti-tubulin antibodies as a control to demonstrate equal amounts of cell lysate. Maximal expression levels of dynwt and dynts were observed 3–4 d after removal of tetracycline and daily replacement of the culture medium. (B) dynwt- or dynts-expressing cells were preincubated for 30 min at either 25 or 38°C and directly thereafter incubated in the presence of 125I-Tf at the same temperature for the indicated times. Internalized 125I-Tf was expressed as a percentage of total cell-associated 125I-Tf (n = 2 ± SD). The results shown are representative of three independent experiments. In comparison with dynwt cells (○), uptake of125I-Tf by dynts cells (□) was strongly reduced at 38°C but not at 25°C.
Figure 3
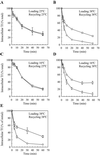
Inhibited TfR recycling by dynts. (A and B) dynwt (○) and dynts (□)-expressing cells were loaded with 125I-Tf for 1 h at either 25°C (A) or 38°C (B). Cell surface-associated 125I-Tf was removed at 0°C, after which recycling of endocytosed125I-Tf was measured during continued incubations at 25°C (A) or 38°C (B). Intracellular 125I-Tf is plotted as a percentage of total endocytosed 125I-Tf (n = 2, mean ± SD). The experiments shown are representative of 13 independent experiments, each with duplicate samples. For kinetic analysis, see Table 1. (C and D) Sorting endosomes of dynwt(○) and dynts (□)-expressing cells were loaded with125I-Tf for 1 h at 16°C. Cell surface-associated125I-Tf was removed at 0°C, and recycling of endocytosed125I-Tf was measured at either 25°C (C) or 38°C (D). Intracellular 125I-Tf is plotted as a percentage of total endocytosed 125I-Tf (n = 2, mean ± SD). The experiments shown are representative of two to five independent experiments, each with duplicate samples. For the kinetic analysis, see Table 1. (E) dynwt (○) and dynts(□)-expressing cells were loaded with 125I-Tf-SS-biotin for 1 h at 38°C. Cell surface-associated125I-Tf-SS-biotin was removed at 0°C, after which recycling of endocytosed 125I-Tf-SS-biotin was allowed at 38°C for the times indicated. The biotin moiety was then removed selectively from surface-exposed 125I-Tf-SS-biotin by exposing the cells to MESNA at 0°C. Intracellular125I-Tf-SS-biotin was then collected from cell lysates and plotted as a percentage of total internalized125I-Tf-SS-biotin (mean ± SD from two independent experiments). For the kinetic analysis, see Table 1.
Figure 4
Inhibition of Tf recycling by dynts is not due to defective endosome acidification. (A) dynwt(circles) and dynts (squares)-expressing cells were loaded with 125I-Tf for 1 h at 38°C. Cell surface-associated 125I-Tf was removed at 0°C by the acidic-neutral wash procedure using either MES buffer (open symbols) or acetate buffer (closed symbols). The cells were then reincubated at 38°C, and the release of 125I-Tf was measured. Intracellular 125I-Tf is plotted as a percentage of total endocytosed 125I-Tf (n = 2, mean ± SD). The experiments shown are representative of three independent experiments, each with duplicate samples. The acetate treatment assured the release of Fe3+ from intracellular 125I-Tf. dynts inhibited the release of 125I-Tf irrespective of acetate or MES treatment. Lack of endosome acidification thus can not explain the results. (B) As a positive control for this procedure, dynwt-expressing cells were loaded for 1 h at 38°C with 125I-Tf in the absence (○) or presence (▵ and □) of 100 nM of concanamycin A, a vacuolar proton pump inhibitor. Cell surface-associated 125I-Tf was removed at 0°C by the acid-neutral wash procedure using either MES buffer (□) or acetate buffer (○ and▵). Cells were then reincubated at 38°C in the absence (○) or presence (▵ and □) of 100 nM concanamycin A and release of 125I-Tf was measured. Intracellular 125I-Tf is plotted as a percentage of total internalized 125I-Tf. The results shown (n = 2, mean ± SD) are representative of five independent experiments. The acetate treatment restored 125I-Tf recycling by concanamycin A-treated cells, illustrating the effectiveness of this procedure.
Figure 5
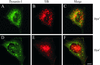
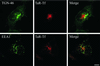
dynts cells redistribute intracellular TfR. dynwt (A–C) and dynts (D–F)-expressing cells were incubated for 30 min at 38°C, fixed, and immuno-double–labeled for dynamin-1 (green) and TfR (red). Integrated views of entire cells were obtained by superimposition of 25 sequential 0.3-μm optical sections. Note that in dynts cells dynamin-1 accumulated at the edge of the cell and in large aggregates. TfR accumulated at the plasma membrane as well as in tubular endosomes in the perinuclear area. The latter structure apparently also accumulated dynts. Bar, 10 μm.
Figure 6
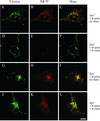
dynts cells accumulate endocytosed Tf in a perinuclear compartment. dynwt (A–F) and dynts (G–L)-expressing cells were pulse-labeled with both TaR-Tf and F-dextran for 1 h at 38°C. Extracellular and cell surface-associated labels were removed at 0°C by the acid-neutral wash procedure. The cells were then either fixed immediately (A–C, G–I) or chased for 1 h at 38°C before fixation (D-F, J-L). dynwt cells released almost all TaR-Tf during the chase, whereas dynts cells retained significant amounts of TaR-Tf in F-dextran lacking endosomes that localize both at the perinuclear area and at the periphery of the cell. Bar, 10 μm.
Figure 7
dynts cells accumulate Tf in recycling endosomes. dynts-expressing cells were loaded with TaR-Tf for 1 h at 38°C. Cell surface-associated TaR-Tf was removed at 0°C by the acid-neutral wash procedure and the cells were chased for 1 h at 38°C, fixed, permeabilized, and immunolabeled for either TGN-46 or EEA1. TaR-Tf was retained in peripheral and perinuclear endosomes that are distinct from the TGN (TGN-46) and sorting endosomes (EEA1). Bar, 10 μm.
Figure 8
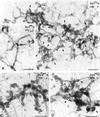
dynts localizes on endosomal clathrin-coated buds. dynwt (A and C) and dynts(B and D) cells were incubated for 1 h at 25°C with Tf/HRP to allow loading of the entire recycling pathway with HRP activity. The cells were then incubated for 5 min at 38°C to initiate the dominant-negative effect of dynts. The cells were processed for whole mount immunoelectron microscopy and labeled for dynamin-1 (dynwt or dynts, 10-nm gold) and clathrin (cla, 5-nm gold; A and B) or dynamin-1 (10-nm gold) and TfR (5-nm gold; C and D). (A) Clathrin-coated buds on tubular endosomes in dynwtcells are largely devoid of dynwt. (B) Clathrin-coated buds on extended tubular endosomal networks in dynts cells are mostly labeled for dynts (for examples, see arrowheads). For quantification, see Figure 9. (C) TfR-labeled tubular endosomes in dynwt cells contain little, if any, dynwt. (D) TfR-labeled endosomes in dynts cells contain many dynts-labeled buds (arrowheads). Limited labeling for TfR on dynts-containing buds was probably due to stearic hindrance. Bars, 200 nm.
Figure 9
Dynamin and clathrin codistribute on endosomal buds. (A) Double-labeled whole mount immunoelectron microscopic preparations of nontransfected HeLa cells (see Figure 1) were analyzed for the presence of endogenous dynamin-2 and clathrin on endosomal buds. Of 300 immunolabeled DAB-positive buds that were counted, ∼93% contained both endogenous dynamin-2 and clathrin. (B) Double-labeled whole mount immunoelectron microscopic preparations of dynts cells (see Figure 8B) were analyzed for the presence of dynts and clathrin on endosomal buds. Of 700 labeled DAB-positive buds that were counted, ∼90% contained both dynts and clathrin.
Figure 10
Interference of TfR recycling by BFA. (A and B) dynwt (circles) and dynts (squares)-expressing cells were loaded with 125I-Tf for 1 h at either 25°C (A) or 38°C (B). Cell surface-associated 125I-Tf was removed at 0°C, and the cells were reincubated at 25°C (A) or 38°C (B) in the absence (open symbols) or presence (closed symbols) of 10 μg/ml BFA, and the release of 125I-Tf was measured. Intracellular 125I-Tf is plotted as a percentage of total endocytosed 125I-Tf (n = 2, mean ± SD). The experiments shown are representative of two independent experiments each with duplicate samples. For the kinetic analysis see Table 1. At 25°C BFA interfered with 125I-Tf recycling by both dynwt and dynts cells. In contrast, at 38°C BFA had no additive effect on 125I-Tf recycling by dynts cells.
Similar articles
- Dynamin- and clathrin-dependent endocytic pathway in unicellular eukaryote Paramecium.
Wiejak J, Surmacz L, Wyroba E. Wiejak J, et al. Biochem Cell Biol. 2004 Oct;82(5):547-58. doi: 10.1139/o04-098. Biochem Cell Biol. 2004. PMID: 15499383 - Endocytosed transferrin receptors recycle via distinct dynamin and phosphatidylinositol 3-kinase-dependent pathways.
van Dam EM, Ten Broeke T, Jansen K, Spijkers P, Stoorvogel W. van Dam EM, et al. J Biol Chem. 2002 Dec 13;277(50):48876-83. doi: 10.1074/jbc.M206271200. Epub 2002 Oct 7. J Biol Chem. 2002. PMID: 12372835 - Aquaporin-2 localization in clathrin-coated pits: inhibition of endocytosis by dominant-negative dynamin.
Sun TX, Van Hoek A, Huang Y, Bouley R, McLaughlin M, Brown D. Sun TX, et al. Am J Physiol Renal Physiol. 2002 Jun;282(6):F998-1011. doi: 10.1152/ajprenal.00257.2001. Am J Physiol Renal Physiol. 2002. PMID: 11997316 - Caveosomes and endocytosis of lipid rafts.
Nichols B. Nichols B. J Cell Sci. 2003 Dec 1;116(Pt 23):4707-14. doi: 10.1242/jcs.00840. J Cell Sci. 2003. PMID: 14600257 Review. - Cargo recognition during clathrin-mediated endocytosis: a team effort.
Sorkin A. Sorkin A. Curr Opin Cell Biol. 2004 Aug;16(4):392-9. doi: 10.1016/j.ceb.2004.06.001. Curr Opin Cell Biol. 2004. PMID: 15261671 Review.
Cited by
- Dynasore Blocks Ferroptosis through Combined Modulation of Iron Uptake and Inhibition of Mitochondrial Respiration.
Clemente LP, Rabenau M, Tang S, Stanka J, Cors E, Stroh J, Culmsee C, von Karstedt S. Clemente LP, et al. Cells. 2020 Oct 9;9(10):2259. doi: 10.3390/cells9102259. Cells. 2020. PMID: 33050207 Free PMC article. - Clathrin plaques and associated actin anchor intermediate filaments in skeletal muscle.
Franck A, Lainé J, Moulay G, Lemerle E, Trichet M, Gentil C, Benkhelifa-Ziyyat S, Lacène E, Bui MT, Brochier G, Guicheney P, Romero N, Bitoun M, Vassilopoulos S. Franck A, et al. Mol Biol Cell. 2019 Mar 1;30(5):579-590. doi: 10.1091/mbc.E18-11-0718. Epub 2019 Jan 2. Mol Biol Cell. 2019. PMID: 30601711 Free PMC article. - Murine cytomegalovirus perturbs endosomal trafficking of major histocompatibility complex class I molecules in the early phase of infection.
Tomas MI, Kucić N, Mahmutefendić H, Blagojević G, Lucin P. Tomas MI, et al. J Virol. 2010 Nov;84(21):11101-12. doi: 10.1128/JVI.00988-10. Epub 2010 Aug 18. J Virol. 2010. PMID: 20719942 Free PMC article. - Arrestin-independent constitutive endocytosis of GPR125/ADGRA3.
Spiess K, Bagger SO, Torz LJ, Jensen KHR, Walser AL, Kvam JM, Møgelmose AK, Daugvilaite V, Junnila RK, Hjortø GM, Rosenkilde MM. Spiess K, et al. Ann N Y Acad Sci. 2019 Nov;1456(1):186-199. doi: 10.1111/nyas.14263. Epub 2019 Oct 29. Ann N Y Acad Sci. 2019. PMID: 31659746 Free PMC article. - Dynamin 2 and human diseases.
Durieux AC, Prudhon B, Guicheney P, Bitoun M. Durieux AC, et al. J Mol Med (Berl). 2010 Apr;88(4):339-50. doi: 10.1007/s00109-009-0587-4. Epub 2010 Feb 3. J Mol Med (Berl). 2010. PMID: 20127478 Review.
References
- Baba T, Ueda H, Terada N, Fujii Y, Ohno S. Immunocytochemical study of endocytotic structures accumulated in HeLa cells transformed with a temperature-sensitive mutant of dynamin. J Histochem Cytochem. 1999;47:637–648. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources