Dendritic spines elongate after stimulation of group 1 metabotropic glutamate receptors in cultured hippocampal neurons - PubMed (original) (raw)
Dendritic spines elongate after stimulation of group 1 metabotropic glutamate receptors in cultured hippocampal neurons
Peter W Vanderklish et al. Proc Natl Acad Sci U S A. 2002.
Abstract
Changes in the morphology of dendritic spines are correlated with synaptic plasticity and may relate mechanistically to its expression and stabilization. Recent work has shown that spine length can be altered by manipulations that affect intracellular calcium, and spine length is abnormal in genetic conditions affecting protein synthesis in neurons. We have investigated how ligands of group 1 metabotropic glutamate receptors (mGluRs) affect spine shape; stimulation of these receptors leads both to calcium release from intracellular stores and to dendritic protein synthesis. Thirty-minute incubation of cultured hippocampal slices and dissociated neurons with the selective group 1 mGluR agonist (S)-3,5-dihydroxyphenylglycine (DHPG) induced a significant increase in the average length of dendritic spines. This elongation resulted mainly from the growth of existing spines and was also seen even in the presence of antagonists of ionotropic receptors, indicating that activation of these receptors by mGluR-induced glutamate release was not required. Prolonged antagonism of group 1 mGluRs with (S)-alpha-methyl-4-carboxyphenylglycine (MCPG) did not result in shorter average spine length. Elongation of dendritic spines induced by DHPG was blocked by calcium chelation and by preincubation with the protein synthesis inhibitor puromycin. The results suggest that in vivo activation of group 1 mGluRs by synaptically released glutamate affects spine shape in a protein synthesis-dependent manner.
Figures
Figure 1
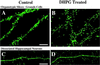
Examples of the effect of mGluR stimulation on the length of dendritic spines in two hippocampal culture systems. Slices (A and B) and dissociated neurons (C and D) were fixed after treatment with vehicle control (Left) or 100 μM DHPG (Right). Control and treated cultures prepared from the same animals were processed in parallel. Scale bar = 5 μm.
Figure 2
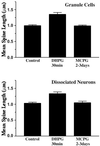
The effect of DHPG on the average length of dendritic spines of dentate gyrus granule cells in slice culture and dissociated hippocampal neurons. A 30-min incubation with 100 μM DHPG resulted in a significant increase in the average length of dendritic spines (1.35 ± 0.06 μm) relative to controls acutely treated with AP5/CNQX (0.99 ± 0.03 μm; n = 10 experiments, 2–3 granule cells per experiment, ≥50 spines per cell; P < 0.001, two-tailed Mann–Whitney test). Similar effects were obtained in dissociated neuronal culture (control 1.03 ± 0.03, DHPG 1.33 ± 0.05; P < 0.001). Prolonged incubation with MCPG did not alter the average length of dendritic spines (dentate granule cells, 0.99 ± 0.03; dissociated neurons, 1.05 ± 0.05).
Figure 3
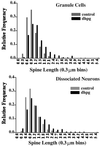
Frequency distribution histograms of dendritic spine length in control and DHPG-treated cultures. (Upper) The length of spines on the dendrites of dentate granule cells in slice culture were measured and segregated into bins of 0.3 μm. The composite frequency distribution from 10 experiments shows that the occurrence of longer spines increases whereas that of shorter spines decreases. (Lower) The same effect is evident in composite frequency distributions of spine lengths in dissociated neuronal cultures.
Figure 4
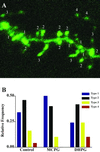
Example of spine types and analysis of shifts in the relative abundance of these types accompanying DHPG treatment. (A) Maximum intensity projection of an image stack taken from a DHPG-treated dentate granule cell. Four spine types are labeled, classified as described in Methods and differentiated with respect to length and shape characteristics. (B) Relative abundance of spine types in control slices and in slices treated acutely with DHPG or chronically for 2–3 days with MCPG (control and DHPG treatments also included AP5 + CNQX). Spines identified as type 3 or 4, characterized by longer, thinner profiles with smaller spine heads, are increased in frequency with DHPG treatment whereas shorter (“nubbin”) spines, type 1, are decreased. A slight shift in the opposite direction with chronic MCPG is seen in this experiment, but this result did not occur reliably enough to influence the average spine length. The proportion of type 2 spines, the classic mushroom-shaped profiles, did not change with DHPG treatment.
Figure 5
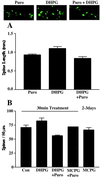
(A) The effect of puromycin on DHPG-induced spine lengthening in hippocampal slice cultures. Mean spine lengths in slices treated with puromycin (Puro), DHPG, and DHPG in the presence of puromycin (DHPG + Puro) are graphed with sample images from each treatment above. (B) The effect of simultaneous manipulation of group 1 mGluRs and protein synthesis on spine density. The average number of dendritic spines per 50-μm segment of secondary dendrites of dentate granule cells was not significantly different from controls (70.9 ± 4.1, n = 7) after treatment with DHPG (82.6 ± 5.1, n = 9) or after 2–3 days of incubation with MCPG (66.5 ± 3.9, n = 11). Addition of puromycin simultaneously with either drug also did not significantly change the density of spines, although a trend toward fewer spines was seen with DHPG + puromycin (56.3 ± 1.0, n = 3; P = 0.06, Mann–Whitney U test, two-tailed).
Similar articles
- Heterologous modulation of inhibitory synaptic transmission by metabotropic glutamate receptors in cultured hippocampal neurons.
Fitzsimonds RM, Dichter MA. Fitzsimonds RM, et al. J Neurophysiol. 1996 Feb;75(2):885-93. doi: 10.1152/jn.1996.75.2.885. J Neurophysiol. 1996. PMID: 8714661 - Group I metabotropic glutamate receptors mediate an inward current in rat substantia nigra dopamine neurons that is independent from calcium mobilization.
Guatteo E, Mercuri NB, Bernardi G, Knöpfel T. Guatteo E, et al. J Neurophysiol. 1999 Oct;82(4):1974-81. doi: 10.1152/jn.1999.82.4.1974. J Neurophysiol. 1999. PMID: 10515987 - Synaptically activated calcium responses in dendrites of hippocampal oriens-alveus interneurons.
Gee CE, Woodhall G, Lacaille JC. Gee CE, et al. J Neurophysiol. 2001 Apr;85(4):1603-13. doi: 10.1152/jn.2001.85.4.1603. J Neurophysiol. 2001. PMID: 11287484 - Dendritic spines for neuroprotection: a hypothesis.
Segal M. Segal M. Trends Neurosci. 1995 Nov;18(11):468-71. doi: 10.1016/0166-2236(95)92765-i. Trends Neurosci. 1995. PMID: 8592749 Review. - Understanding regulation of nerve cell death by mGluRs as a method for development of successful neuroprotective strategies.
Baskys A, Blaabjerg M. Baskys A, et al. J Neurol Sci. 2005 Mar 15;229-230:201-9. doi: 10.1016/j.jns.2004.11.028. Epub 2004 Dec 15. J Neurol Sci. 2005. PMID: 15760640 Review.
Cited by
- FMRP regulates an ethanol-dependent shift in GABABR function and expression with rapid antidepressant properties.
Wolfe SA, Workman ER, Heaney CF, Niere F, Namjoshi S, Cacheaux LP, Farris SP, Drew MR, Zemelman BV, Harris RA, Raab-Graham KF. Wolfe SA, et al. Nat Commun. 2016 Sep 26;7:12867. doi: 10.1038/ncomms12867. Nat Commun. 2016. PMID: 27666021 Free PMC article. - Cortical regulation of dopamine depletion-induced dendritic spine loss in striatal medium spiny neurons.
Neely MD, Schmidt DE, Deutch AY. Neely MD, et al. Neuroscience. 2007 Oct 26;149(2):457-64. doi: 10.1016/j.neuroscience.2007.06.044. Epub 2007 Jul 17. Neuroscience. 2007. PMID: 17888581 Free PMC article. - Reversible inhibition of PSD-95 mRNA translation by miR-125a, FMRP phosphorylation, and mGluR signaling.
Muddashetty RS, Nalavadi VC, Gross C, Yao X, Xing L, Laur O, Warren ST, Bassell GJ. Muddashetty RS, et al. Mol Cell. 2011 Jun 10;42(5):673-88. doi: 10.1016/j.molcel.2011.05.006. Mol Cell. 2011. PMID: 21658607 Free PMC article. - Activity-dependent FUS dysregulation disrupts synaptic homeostasis.
Sephton CF, Tang AA, Kulkarni A, West J, Brooks M, Stubblefield JJ, Liu Y, Zhang MQ, Green CB, Huber KM, Huang EJ, Herz J, Yu G. Sephton CF, et al. Proc Natl Acad Sci U S A. 2014 Nov 4;111(44):E4769-78. doi: 10.1073/pnas.1406162111. Epub 2014 Oct 16. Proc Natl Acad Sci U S A. 2014. PMID: 25324524 Free PMC article. - Current advances in local protein synthesis and synaptic plasticity.
Pfeiffer BE, Huber KM. Pfeiffer BE, et al. J Neurosci. 2006 Jul 5;26(27):7147-50. doi: 10.1523/JNEUROSCI.1797-06.2006. J Neurosci. 2006. PMID: 16822970 Free PMC article. Review.
References
- Lynch G. Neurobiol Learn Mem. 1998;70:82–100. - PubMed
- Calverley R K, Jones D G. Brain Res Brain Res Rev. 1990;15:215–249. - PubMed
- Korkotian E, Segal M. Neuroreport. 1999;10:2875–2877. - PubMed
- Maletic-Savatic M, Malinow R, Svoboda K. Science. 1999;283:1923–1927. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources