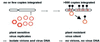Endogenous viral sequences and their potential contribution to heritable virus resistance in plants - PubMed (original) (raw)
Endogenous viral sequences and their potential contribution to heritable virus resistance in plants
M F Mette et al. EMBO J. 2002.
Abstract
Tobacco endogenous pararetroviruses (TEPRVs) represent the first virus-derived repetitive sequence family found in plants. The sequence conservation of TEPRVs and the lack of an exogenous form of the virus suggest that TEPRVs serve a beneficial function, perhaps by furnishing virus resistance via homologous sequence interactions. This hypothesis is supported by the observation that TEPRVs are methylated and negligibly transcribed. Moreover, transgenes driven by the TEPRV enhancer are silenced and methylated when introduced into tobacco, but remain active and unmethylated in non-host species devoid of sequences homologous to TEPRVs. In transgenic Arabidopsis, the TEPRV enhancer is active primarily in shoot meristems. This suggests that the virus giving rise to TEPRVs could infect germ cell precursors, a prerequisite for meiotically heritable insertions into host chromosomes. The copy number, organization and methylation of TEPRVs in tetraploid tobacco and one of its diploid ancestors, Nicotiana sylvestris, the presumed original host for the virus, have remained constant since polyploid formation. The remarkable conservation of these features in two independently evolving species further supports a role for TEPRVs in viral immunity.
Figures
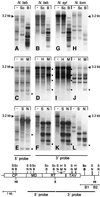
Fig. 1. Methylation of TEPRVs. Total DNA isolated from tetraploid tobacco (N. tab.) and its two diploid ancestors, N.sylvestris (N. syl.) and N.tomentosiformis (N. tom.), was digested with _Xba_I (X) and _Hin_dIII (Hi; ‘–’ lane of each panel; arrowheads indicate 3.2 kb bands produced by this digest) or with X, Hi and one member of three isoschizomer pairs of methylation-sensitive/insensitive restriction enzymes: _Scr_FI–_Bst_NI (Sc and B; blots A, B, G and H); _Hpa_II–_Msp_I (H and M; blots C, D, I and J) or _Sau_3AI–_Nde_II (S and N; blots E, F, K and L). These enzymes have recognition sequences of CCWGG, CCGG and GATC, respectively (methylation at bold Cs inhibits digestion by the first enzyme in each pair). Dots to the right of H/M and S/N blots indicate positions of new fragments following digestion with M or N. The map shows the ORFs of the putative tobacco pararetrovirus (Jakowitsch et al., 1999), the positions of restriction enzyme sites (twin parallel bars indicate two closely spaced sites for the indicated enzyme pair) and the 5′ and 3′ probes used. Blots (A), (C) and (E) were probed with the 5′ region; the remaining blots with the 3′ region. CP, coat protein; MD, movement domain; RT, reverse transcriptase; TAV, _trans_-activating domain.
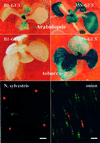
Fig. 2. Identification of the TEPRV enhancer. To identify the enhancer element of the putative tobacco pararetrovirus giving rise to TEPRVs, three fragments (B1, B12 and B2) that are downstream of the last major ORF (TAV) of the putative viral genome were placed upstream of a minimal promoter (TATA), which is negligibly active in plants, adjacent to a GUS reporter gene. GUS expression was tested in stably transformed Arabidopsis plants and in transformed tobacco calli, all of which were pre-screened for kanamycin resistance and NOP, demonstrating that both flanking marker genes (NPTII and NOS) were present and expressed. Plus signs indicate fragments displaying enhancer activity in the plant material indicated. The actual numbers of GUS-positive transformants obtained with each construct over the total number of KanR NOP+ transformants tested were as follows. Arabidopsis: TATA, 0/15; B2, 0/19; B12, 20/24; B1, 16/19. Tobacco: TATA, 0/4; B2, n.d.; B12, 0/11; B1, 0/12. TAV, _trans_-activating domain; NPTII, neomycinphosphotransferase; NOS, nopaline synthase.
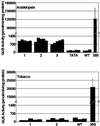
Fig. 3. Quantification of GUS activity in B1–GUS transgenic Arabidopsis and tobacco seedlings. GUS activity was measured in whole seedling extracts from three (Arabidopsis) or two (tobacco) independent transgenic lines containing the B1–GUS construct (data for five seedlings/line are shown). Segregation analysis of kanamycin resistance demonstrated that all lines contained a single transgene locus; DNA blotting using a GUS probe detected only a single band of the size expected for an intact B1–GUS expression cassette in all lines (data not shown). Seedlings containing a 35S–GUS transgene were used as positive controls (average of three separate determinations; bars indicate standard deviations). Three seedlings from lines not containing a GUS gene (WT) were used as a negative controls; for Arabidopsis, an additional control included five seedlings from a line containing only a minimal promoter (TATA)–GUS chimeric transgene. Lack of activity of this construct has been demonstrated previously in tobacco seedlings (Matzke et al., 2001). Leaves of adult tobacco plants transformed with B1–GUS or B12–GUS transgenes (several independent lines tested for each construct) were negative for GUS activity (data not shown).
Fig. 4. Shoot meristem activity of the B1 enhancer in Arabidopsis and transient expression in onion and N.sylvestris. Top left: shoot meristem activity of B1–GUS transgenes in Arabidopsis seedlings. Middle left: B1–GUS tobacco seedlings did not exhibit GUS expression in any tissue. 35S–GUS transgenes display fairly constitutive expression in Arabidopsis (top right) and tobacco (middle right). Bottom: transient expression of smRS-GFP and DsRed1 expression in onion epidermal cells (right) and N.sylvestris petal cells (left) co-bombarded with B1–smRS-GFP (green) and 35S–DsRed1 (red) constructs. Bar = 0.1 mm.
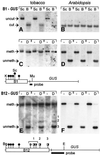
Fig. 5. Methylation analysis of B1–GUS and B12–GUS transgenes in tobacco and Arabidopsis. Methylation was analyzed in the B1 and B12 enhancer regions of GUS transgenes using Scr_FI–_Bst_NI (CCWGG) and Dde_I (CTNAG; methylation at the bold Cs inhibits enzyme activity). There is one site for Scr_FI–_Bst_NI in the B1 fragment (circle labeled ‘Sc’, B1–_GUS map) and none in the B2 fragment. There are four sites for Dde_I in the B1 fragment (unlabeled filled circles, B1–_GUS map) and an additional five in the B2 fragment (unlabeled partially filled circles and squares, B12–_GUS map). Digests were performed on total DNA from three independent lines of tobacco or Arabidopsis containing single copies of the B1–_GUS (blots A–D) or B12–GUS (blots E and F) constructs (determined by segregation of kanamycin resistance and Southern blot analysis of the GUS expression cassette; data not shown). (A and B) Double digestion with _Kpn_I (K)–_Mun_I (Mu) and digestion with either _Scr_FI (Sc; methylation sensitive) or _Bst_NI (B; methylation insensitive). (C and D) Double digestion with K–Mu and either minus (‘–’ lanes) or plus _Dde_I (D lanes). The faint band slightly below the major band in (C), lanes D, suggests incomplete methylation at one of the upstream _Dde_I sites. (E and F) Double digestion with _Bgl_II (Bg)–_Eco_RV (E) and either minus (‘–’ lanes) or plus _Dde_I (D lanes). Blots were probed with GUS coding sequences (black bars). Little or no methylation was detected in Arabidopsis. The filled and partially filled lollipops indicate methylation and partial methylation at the respective sites in tobacco. For _Dde_I, circles denote sites in which the ‘N’ in the recognition sequence is G; squares indicate sites in which N is not G. The numbered bands in (E) correspond to partial methylation at the correspondingly numbered _Dde_I sites on the map. The fragments produced by cutting at sites under the number 1 bracket are not resolved on the gel system used.
Fig. 6. Model for heritable homology-dependent virus resistance conferred by EPRVs. Originally, no EPRVs are present in host chromosomes (left). The virus can infect the plant and free virions and virus DNA are detectable. Over evolutionary time, viral sequences integrate randomly by illegitimate recombination into host chromosomes. Once a threshold copy number is reached (∼500–1000), EPRVs become methylated (filled circles) and silenced through a homology-dependent gene silencing mechanism. Methylated EPRVs can trigger methylation and silencing of free viral genomes through a homology-dependent process involving DNA–DNA or RNA–DNA interactions. Consequently, virus replication is blocked and the host has gained long-term viral immunity.
Similar articles
- A distinct endogenous pararetrovirus family in Nicotiana tomentosiformis, a diploid progenitor of polyploid tobacco.
Gregor W, Mette MF, Staginnus C, Matzke MA, Matzke AJ. Gregor W, et al. Plant Physiol. 2004 Mar;134(3):1191-9. doi: 10.1104/pp.103.031112. Epub 2004 Feb 26. Plant Physiol. 2004. PMID: 14988473 Free PMC article. - Studies on the effects of a flanking repetitive sequence on the expression of single-copy transgenes in Nicotiana sylvestris and in N. sylvestris-N. tomentosiformis hybrids.
Kunz C, Narangajavana J, Jakowitsch J, Park YD, Delon TR, Kovarik A, Koukalová B, van der Winden J, Moscone E, Aufsatz W, Mette MF, Matzke M, Matzke AJ. Kunz C, et al. Plant Mol Biol. 2003 May;52(1):203-15. doi: 10.1023/a:1023937006311. Plant Mol Biol. 2003. PMID: 12825700 - Integration of multiple repeats of geminiviral DNA into the nuclear genome of tobacco during evolution.
Bejarano ER, Khashoggi A, Witty M, Lichtenstein C. Bejarano ER, et al. Proc Natl Acad Sci U S A. 1996 Jan 23;93(2):759-64. doi: 10.1073/pnas.93.2.759. Proc Natl Acad Sci U S A. 1996. PMID: 8570630 Free PMC article. - Integrated pararetroviral sequences define a unique class of dispersed repetitive DNA in plants.
Jakowitsch J, Mette MF, van Der Winden J, Matzke MA, Matzke AJ. Jakowitsch J, et al. Proc Natl Acad Sci U S A. 1999 Nov 9;96(23):13241-6. doi: 10.1073/pnas.96.23.13241. Proc Natl Acad Sci U S A. 1999. PMID: 10557305 Free PMC article. - Viral sequences integrated into plant genomes.
Harper G, Hull R, Lockhart B, Olszewski N. Harper G, et al. Annu Rev Phytopathol. 2002;40:119-36. doi: 10.1146/annurev.phyto.40.120301.105642. Epub 2002 Feb 20. Annu Rev Phytopathol. 2002. PMID: 12147756 Review.
Cited by
- Three infectious viral species lying in wait in the banana genome.
Chabannes M, Baurens FC, Duroy PO, Bocs S, Vernerey MS, Rodier-Goud M, Barbe V, Gayral P, Iskra-Caruana ML. Chabannes M, et al. J Virol. 2013 Aug;87(15):8624-37. doi: 10.1128/JVI.00899-13. Epub 2013 May 29. J Virol. 2013. PMID: 23720724 Free PMC article. - A distinct endogenous pararetrovirus family in Nicotiana tomentosiformis, a diploid progenitor of polyploid tobacco.
Gregor W, Mette MF, Staginnus C, Matzke MA, Matzke AJ. Gregor W, et al. Plant Physiol. 2004 Mar;134(3):1191-9. doi: 10.1104/pp.103.031112. Epub 2004 Feb 26. Plant Physiol. 2004. PMID: 14988473 Free PMC article. - A single unidirectional piRNA cluster similar to the flamenco locus is the major source of EVE-derived transcription and small RNAs in Aedes aegypti mosquitoes.
Aguiar ERGR, de Almeida JPP, Queiroz LR, Oliveira LS, Olmo RP, de Faria IJDS, Imler JL, Gruber A, Matthews BJ, Marques JT. Aguiar ERGR, et al. RNA. 2020 May;26(5):581-594. doi: 10.1261/rna.073965.119. Epub 2020 Jan 29. RNA. 2020. PMID: 31996404 Free PMC article. - Allopolyploidy and the evolution of plant virus resistance.
Gottula J, Lewis R, Saito S, Fuchs M. Gottula J, et al. BMC Evol Biol. 2014 Jul 3;14:149. doi: 10.1186/1471-2148-14-149. BMC Evol Biol. 2014. PMID: 24992820 Free PMC article. - Frontiers in the Standardization of the Plant Platform for High Scale Production of Vaccines.
Citiulo F, Crosatti C, Cattivelli L, Biselli C. Citiulo F, et al. Plants (Basel). 2021 Sep 2;10(9):1828. doi: 10.3390/plants10091828. Plants (Basel). 2021. PMID: 34579360 Free PMC article. Review.
References
- Ashby M.K., Warry,A., Bejarano,E., Khashoggi,A., Burrell,M. and Lichtenstein,C.P. (1997) Analysis of multiple copies of geminiviral DNA in the genome of four closely related Nicotiana species suggests a unique integration event. Plant Mol. Biol., 35, 313–321. - PubMed
- Best S., le Tissier,P.R. and Stoye,J.P. (1997) Endogenous retroviruses and the evolution of resistance to retroviral infection. Trends Microbiol., 5, 313–339. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources