GABAergic innervation organizes synaptic and extrasynaptic GABAA receptor clustering in cultured hippocampal neurons - PubMed (original) (raw)
GABAergic innervation organizes synaptic and extrasynaptic GABAA receptor clustering in cultured hippocampal neurons
Sean B Christie et al. J Neurosci. 2002.
Abstract
We have studied the effects of GABAergic innervation on the clustering of GABA(A) receptors (GABA(A)Rs) in cultured hippocampal neurons. In the absence of GABAergic innervation, pyramidal cells form small (0.36 +/- 0.01 micrometer diameter) GABA(A)R clusters at their surface in the dendrites and soma. When receiving GABAergic innervation from glutamic acid decarboxylase-containing interneurons, pyramidal cells form large (1.62 +/- 0.08 micrometer breadth) GABA(A)R clusters at GABAergic synapses. This is accompanied by a disappearance of the small GABA(A)R clusters in the local area surrounding each GABAergic synapse. Although the large synaptic GABA(A)R clusters of any neuron contained all GABA(A)R subunits and isoforms expressed by that neuron, the small clusters not localized at GABAergic synapses showed significant heterogeneity in subunit and isoform composition. Another difference between large GABAergic and small non-GABAergic GABA(A)R clusters was that a significant proportion of the latter was juxtaposed to postsynaptic markers of glutamatergic synapses such as PSD-95 and AMPA receptor GluR1 subunit. The densities of both the glutamate receptor-associated and non-associated small GABA(A)R clusters were decreased in areas surrounding GABAergic synapses. However, no effect on the density or distribution of glutamate receptor clusters was observed. The results suggest that there are local signals generated at GABAergic synapses that induce both assembly of large synaptic GABA(A)R clusters at the synapse and disappearance of the small GABA(A)R clusters in the surrounding area. In the absence of GABAergic innervation, weaker GABA(A)R-clustering signals, generated at glutamatergic synapses, induce the formation of small postsynaptic GABA(A)R clusters that remain juxtaposed to glutamate receptors at glutamatergic synapses.
Figures
Fig. 1.
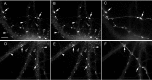
GABAergic innervation induces the formation of large GABAAR clusters at GABAergic synapses. Smaller GABAAR clusters are also present outside GABAergic synapses. Hippocampal neurons after 19–22 d in culture were immunolabeled with the rabbit anti-GABAA receptor subunit γ2 (A, D, G,J), mouse monoclonal anti-gephyrin (B, E), sheep anti-GAD (C,F, I, L), guinea pig anti-α1 (H), or mouse monoclonal anti-β2/3 (K).D_–_F show at high magnification the fields in A_–_C corresponding to the_boxed area_ in A, respectively. Large clusters of GABAA receptors (D,G, H, J, K,arrows) and gephyrin (E,arrows) colocalize with GAD-positive boutons (F, I, L,arrows), whereas small clusters of GABAARs (D, G, H,J, K, filled arrowheads) colocalize with small clusters of gephyrin (E,filled arrowheads) but not with GAD boutons. A number of small GABAAR clusters not colocalizing with GAD contained only one of the two subunit classes (G,H, J, K, empty arrowheads). Scale bar (shown in A):A_–_C, 10 μm; (shown in_D_): D_–_L, 5 μm.
Fig. 2.
Various α subunit isoforms of the GABAAR colocalize in all large GABAergic synaptic clusters, but not in all smaller non-GABAergic clusters. Hippocampal pyramidal neurons were triple labeled with the GABAA subunit isoform-specific antibodies guinea pig anti-α1(A, D), rabbit anti-α2(B), or rabbit anti-α3(E) in conjunction with sheep anti-GAD (C, F). All of the larger GAD-colocalizing GABAAR clusters (A,B, D, E,arrows) and many smaller clusters (A,B, D, E, filled arrowheads) that do not colocalize GAD show the presence of the two α subunit isoforms. However, a significant population of the smaller clusters contained only one of the two α−subunit isoforms (A, B, D,E, empty arrowheads). Neurons were cultured for 19 d. Scale bar (shown in A): 5 μm.
Fig. 3.
Various β subunit isoforms of the GABAAR colocalize in all large GABAergic synaptic clusters and most small non-GABAergic clusters. Cultured hippocampal neurons were triple labeled with the GABAAR subunit isoform-specific antibodies rabbit anti-β1(A), mouse monoclonal (62–3G1) anti-β2/3 (B, E,H), rabbit anti-β2(D), and rabbit anti-β3(G) in conjunction with sheep anti-GAD (C, F). All of the larger GAD-colocalizing GABAAR subunit isoform clusters (A, B, D,E, arrows) and many smaller clusters of β1-IL and β2/3 (A,B, filled arrowheads) or β3and β2/3 (G, H,arrows) that do not colocalize GAD show colocalization of the two β-subunit isoforms. β2 expression was observed only within GAD-positive interneurons (F,filled arrowheads). Some small GABAAR clusters contained only one of the two β-subunit isoforms examined (A, B, G,H, empty arrowheads). Neurons shown in_A_–F were cultured for 19 d; neuron in G and H was cultured for 28 d.A_–_C, G, and_H_ show the processes of pyramidal neurons;D_–_F show the processes of an interneuron. Scale bar (shown in A): 5 μm.
Fig. 4.
Large clusters of GABAAR accumulate postsynaptically to active GABAergic presynaptic boutons and show exo-endocytosis of synaptic vesicles. Hippocampal cultures were triple labeled with guinea pig anti-γ2 subunit (A), rabbit anti-VGAT (B), and sheep anti-GAD (C) antibodies. VGAT and GAD concentrate presynaptically in the interneuronal varicosities (A, B, arrows) contacting a pyramidal neuron and colocalize with large postsynaptic clusters of the γ2 subunit-containing GABAARs (arrows) but do not colocalize with the small clusters of GABAARs (A_–_C,filled arrowheads). In live cell labeling conditions for the synaptic vesicle exo-endocytotic assay (D_–_I), active synapses were labeled with the Syt-N anti-synaptotagmin antibody at 37°C (E, arrows). However, when live cells were incubated at 4°C (to prevent exocytosis and endocytosis), no labeling of synaptotagmin with the Syt-N antibody was observed (H, arrows). After live cell labeling with the Syt-N antibodies, cells were fixed and incubated with guinea pig anti-γ2 subunit and sheep anti-GAD to reveal the localization of large GABAAR clusters (D,G, arrows) and GAD-containing presynaptic terminals (F, I, arrows). Note that the Syt-N labeling of active synapses occurs in GAD-containing terminals and colocalizes with large GABAAR clusters (D, E, F,arrows). Neurons in A_–_C_were cultured for 21 d, and neurons in_D_–_I were cultured for 28 d. Scale bar (shown in A): 5 μm.
Fig. 5.
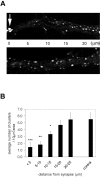
GABAergic innervation induces a reduction in the density of small clusters in dendritic areas adjacent to GABAergic synapses. A, Dendrite segments (25 μm long) from two dendrites from the same pyramidal neuron, one receiving GABAergic innervation (top panel) and another dendrite not receiving GABAergic innervation (bottom panel). GABAAR clusters were visualized with a rabbit anti-γ2 antibody. Note the presence of a large GABAAR cluster (arrow in top panel) at the GABAergic synapse (identified by the colocalization of a GAD-containing bouton), and the lower density of small GABAAR clusters in the adjacent area (noticeable up to 15 μm distance). B, The graph shows that the reduction of the density of small clusters is significant up to 15 μm from the synapse. Beyond 15 μm, the density of the clusters is similar to that of dendrites not receiving GABAergic innervation (control). Quantification of the average cluster density around the synapse was done in five 5 μm zones distal to the site of a GABAergic synapse and compared with non-GABAergic innervated dendritic areas of the same neuron in 22-d-old cultures. (***p < 0.005, **p < 0.01, *p < 0.05; n = 6 matched dendrite pairs).
Fig. 6.
A population of small GABAAR clusters are associated with glutamatergic synaptic markers. GABAergic innervation reduces the local density of small GABAAR clusters that associate with glutamatergic synapses within pyramidal neurons. Hippocampal neurons were labeled with rabbit anti-γ2 (A), mouse monoclonal anti-PSD-95 (B), mouse monoclonal anti-β2/3 (D), rabbit anti-GluR1 (E), guinea pig anti-α1(G), and mouse monoclonal SV2 (H) in conjunction with GAD (C, F, I). The large GABAAR clusters colocalized with GAD and SV2 in GABAergic synapses (A_–_I,arrows). Some small GABAA subunit clusters that were not associated with GAD were associated with PSD-95 (A, B, filled arrowheads), GluR1 (D, E, filled arrowheads), and SV2 (G, H,filled arrowheads). Some small GABAAR clusters were not associated with glutamatergic markers (A, B, G,H, empty arrowheads). A comparison between processes of the same neuron (the two lower horizontal dendrites in D_–_F) shows that the association between small GABAAR clusters and GluR1 clusters in dendritic spines observed in the top dendrite that does not receive GABAergic innervation (D,E, filled arrowheads) is not seen in the GluR1-containing spines of the bottom dendrite that receives GABAergic innervation (D, E, empty arrowheads). Scale bar (shown in A): 5 μm.
Fig. 7.
Clusters of glutamatergic postsynaptic density protein PSD-95 can be apposed to presynaptic and postsynaptic GABAergic synaptic markers, but they remain segregated from the GABAergic markers. Hippocampal cultures were labeled with rabbit anti-γ2 (A), mouse monoclonal anti-PSD-95 (B), and sheep anti-GAD (C). Large clusters of GABAAR γ2 subunit colocalize with GAD-positive presynaptic boutons (arrows). A single GABAergic synapse (A_–_C, arrows) has been magnified in the insets to show detail. Note colocalization of GAD terminal and the GABAAR clusters and how the presynaptic GAD and postsynaptic GABAAR clusters “avoid” the PSD-95 clusters (empty arrowheads) by taking a digitated shape, preserving the segregation of the two postsynaptic receptor clusters. Neurons were cultured for 21 d. Scale bar (shown in A): 5 μm; _inset_scale bar, 1 μm.
Similar articles
- Mismatched appositions of presynaptic and postsynaptic components in isolated hippocampal neurons.
Rao A, Cha EM, Craig AM. Rao A, et al. J Neurosci. 2000 Nov 15;20(22):8344-53. doi: 10.1523/JNEUROSCI.20-22-08344.2000. J Neurosci. 2000. PMID: 11069941 Free PMC article. - Disruption of postsynaptic GABA receptor clusters leads to decreased GABAergic innervation of pyramidal neurons.
Li RW, Yu W, Christie S, Miralles CP, Bai J, Loturco JJ, De Blas AL. Li RW, et al. J Neurochem. 2005 Nov;95(3):756-70. doi: 10.1111/j.1471-4159.2005.03426.x. J Neurochem. 2005. PMID: 16248887 - Discovering the Intriguing Properties of Extrasynaptic γ-Aminobutyric Acid Type A Receptors.
Orser BA. Orser BA. Anesthesiology. 2024 Jun 1;140(6):1192-1200. doi: 10.1097/ALN.0000000000004949. Anesthesiology. 2024. PMID: 38624275 Review. - GABA(A) receptor trafficking and its role in the dynamic modulation of neuronal inhibition.
Jacob TC, Moss SJ, Jurd R. Jacob TC, et al. Nat Rev Neurosci. 2008 May;9(5):331-43. doi: 10.1038/nrn2370. Nat Rev Neurosci. 2008. PMID: 18382465 Free PMC article. Review.
Cited by
- The effect of sevoflurane and isoflurane anesthesia on single unit and local field potentials.
Aksenov DP, Miller MJ, Dixon CJ, Wyrwicz AM. Aksenov DP, et al. Exp Brain Res. 2019 Jun;237(6):1521-1529. doi: 10.1007/s00221-019-05528-9. Epub 2019 Mar 27. Exp Brain Res. 2019. PMID: 30919011 Free PMC article. - Bergmann glia GABA(A) receptors concentrate on the glial processes that wrap inhibitory synapses.
Riquelme R, Miralles CP, De Blas AL. Riquelme R, et al. J Neurosci. 2002 Dec 15;22(24):10720-30. doi: 10.1523/JNEUROSCI.22-24-10720.2002. J Neurosci. 2002. PMID: 12486165 Free PMC article. - Gephyrin expression and clustering affects the size of glutamatergic synaptic contacts.
Yu W, De Blas AL. Yu W, et al. J Neurochem. 2008 Feb;104(3):830-45. doi: 10.1111/j.1471-4159.2007.05014.x. J Neurochem. 2008. PMID: 18199120 Free PMC article. - Recruitment of Plasma Membrane GABA-A Receptors by Submembranous Gephyrin/Collybistin Clusters.
George S, Chiou TT, Kanamalla K, De Blas AL. George S, et al. Cell Mol Neurobiol. 2022 Jul;42(5):1585-1604. doi: 10.1007/s10571-021-01050-1. Epub 2021 Feb 5. Cell Mol Neurobiol. 2022. PMID: 33547626 - In vivo transgenic expression of collybistin in neurons of the rat cerebral cortex.
Fekete CD, Goz RU, Dinallo S, Miralles CP, Chiou TT, Bear J Jr, Fiondella CG, LoTurco JJ, De Blas AL. Fekete CD, et al. J Comp Neurol. 2017 Apr 1;525(5):1291-1311. doi: 10.1002/cne.24137. Epub 2016 Nov 21. J Comp Neurol. 2017. PMID: 27804142 Free PMC article.
References
- Araujo F, Tan S, Ruano D, Schoemaker H, Benavides J, Vitorica J. Molecular and pharmacological characterization of native cortical gamma-aminobutyric acidA receptors containing both alpha1 and alpha3 subunits. J Biol Chem. 1996;271:27902–27911. - PubMed
- Araujo F, Ruano D, Vitorica J. Native gamma-aminobutyric acid type A receptors from rat hippocampus, containing both alpha 1 and alpha 5 subunits, exhibit a single benzodiazepine binding site with alpha 5 pharmacological properties. J Pharmacol Exp Ther. 1999;290:989–997. - PubMed
- Bacci A, Coco S, Pravettoni E, Schenk U, Armano S, Frassoni C, Verderio C, De Camilli P, Matteoli M. Chronic blockade of glutamate receptors enhances presynaptic release and downregulates the interaction between synaptophysin-synaptobrevin–vesicle-associated membrane protein 2. J Neurosci. 2001;21:6588–6596. - PMC - PubMed
- Backus KH, Arigoni M, Drescher U, Scheurer L, Malherbe P, Mohler H, Benson JA. Stoichiometry of a recombinant GABAA receptor deduced from mutation-induced rectification. NeuroReport. 1993;5:285–288. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources