N-myc promotes survival and induces S-phase entry of postmitotic sympathetic neurons - PubMed (original) (raw)
N-myc promotes survival and induces S-phase entry of postmitotic sympathetic neurons
Kirmo Wartiovaara et al. J Neurosci. 2002.
Abstract
In most postmitotic neurons, expression or activation of proteins that stimulate cell cycle progression or DNA replication results in apoptosis. One potential exception to this generalization is neuroblastoma (NB), a tumor derived from the sympathoadrenal lineage. NBs often express high levels of N-myc, a proto-oncogene that can potently activate key components of the cell cycle machinery. Here, we show that in postmitotic sympathetic neurons, N-myc can induce S-phase entry while protecting neurons from death caused by aberrant cell cycle reentry. Specifically, these experiments demonstrate that expression of N-myc at levels similar to those in NBs caused sympathetic neurons to reenter S-phase, as monitored by 5-bromo-2-deoxyuridine incorporation and expression of cell cycle regulatory proteins, and rescued them from apoptosis induced by withdrawal of their obligate survival factor, nerve growth factor. The N-myc-induced cell cycle entry, but not enhanced survival, was inhibited by coexpression of a constitutively hypophosphorylated form of the retinoblastoma tumor suppressor protein, suggesting that these two effects of N-myc are mediated by separate pathways. In contrast, N-myc did not cause S-phase entry in postmitotic cortical neurons. Thus, N-myc both selectively causes sympathetic neurons to reenter the cell cycle and protects them from apoptosis, potentially contributing to their transformation to NBs.
Figures
Fig. 1.
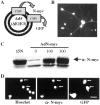
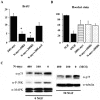
Characterization of a recombinant adenovirus expressing human N-myc. A, Adenovirus construct encoding human N-myc and green fluorescent protein under separate but identical CMV promoters. B, Adenovirus N-myc (AdN)-myc is expressed in sympathetic neurons. Fluorescent micrograph of GFP expression in postmitotic sympathetic neurons 72 hr after infection with 300 MOI of AdN-myc. C, Western blot of equal amounts of protein isolated from LAN-1–15N neuroblastoma cells and from sympathetic neurons infected for 48 hr with 0–300 MOI of N-myc adenovirus and probed with an antibody for N-myc. Note that uninfected sympathetic neurons express N-myc and that LAN-1–15N cells express levels of N-myc similar to sympathetic neurons infected with 100–300 MOI of the N-myc adenovirus. D, Photomicrographs of sympathetic neurons infected with AdN-myc and then analyzed for expression of GFP and immunocytochemically for expression of N-myc (α-N-myc). Cells were also stained with the nuclear dye Hoechst 33258 to identify all of the cells in the field. Note that cells that are GFP positive are also overexpressing N-myc and that the overexpressed N-myc is primarily nuclear. Scale bar, 50 μm.
Fig. 2.
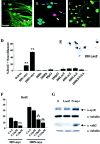
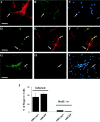
Overexpression of N-myc in postmitotic sympathetic neurons leads to S-phase entry.A_–_C, Photomicrographs of sympathetic neurons infected with recombinant adenovirus, pulse-labeled with BrdU, and then immunocytochemically analyzed for expression of neurofilament-M (green) and BrdU (red). In some cases, cells were also labeled with Hoechst to label all nuclei (blue). A, AdLacZ-expressing neurons do not incorporate BrdU. Neurons were infected with 200 MOI of a β-galactosidase adenovirus and maintained in 10 ng/ml NGF for 3 d. Note that none of the neurofilament-positive neurons are BrdU positive (arrows) and that the only BrdU-positive cells are neurofilament-negative non-neuronal cells (arrowheads).B, Uninfected neurons that were withdrawn from NGF in the presence of BrdU for 30 hr and then analyzed immunocytochemically. Note that a neurofilament-negative non-neuronal cell with an intact nucleus has incorporated BrdU (arrowhead) but that neurons in different phases of apoptosis are not BrdU positive (arrows). C, AdN-myc-expressing neurons incorporate BrdU. Neurons were infected with 200 MOI of N-myc adenovirus and maintained in the presence of NGF. One day later BrdU was added, and cells were analyzed immunocytochemically 48 hr later. Note that under these conditions, many of the neurofilament-positive neurons are BrdU positive (arrows). Scale bar:A, C, 100 μm; B, 50 μm. D, Quantitation of the percentage of cells positive for both BrdU and neurofilament after infection with various MOIs of recombinant adenovirus in the presence of NGF for 1 d, followed by the addition of BrdU for an additional 48 hr. Neurons were infected with recombinant adenoviruses expressing N-myc, constitutively hypophosphorylated pRb (Rb) (Toma et al., 2000), p53 (Slack et al., 1996), β-galactosidase (LacZ), or a kinase-inactive mutant of TrkA (KDTrkA) (Vaillant et al., 1999). Values derive from counts of at least 300 cells in four or more random microscope fields per condition, and error bars represent the SD of the mean. Similar results were obtained in four independent experiments. **p < 0.01; Student's_t_ test. Note that only N-myc led to BrdU incorporation in sympathetic neurons. E, Staining for β-galactosidase (LacZ) in cultures of sympathetic neurons transduced with 200 MOI of the β-galactosidase adenovirus. Note that most of the sympathetic neurons are positively stained. F, Inhibition of N-myc stimulated cell cycle entry by DNA polymerase inhibition. Percentage of BrdU-positive neurons 48 hr after infection with 50 or 200 MOI AdN-myc, cultured in the presence of BrdU with or without 4, 8, or 16 μ
m
cytosine arabinoside for the final 24 hr. Quantitation was performed as in D. Similar results were obtained in three independent experiments. Concentrations of CA shown here did not increase apoptotic cell morphology over the time of the experiment. **p < 0.05; ***p < 0.001.G, AdN-myc expression increases the levels of the S-phase markers cyclin E and cdk2. Western blot analysis of equal amounts of protein from sympathetic neurons that were uninfected (0) or infected with 200 MOI of adenoviruses expressing either β-galactosidase (LacZ) or N-myc for 2 d. Blots were reprobed for total α-tubulin as a loading control. Similar results were obtained in two independent experiments.
Fig. 3.
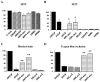
N-myc promotes sympathetic neuron survival after NGF withdrawal. A, AdN-myc does not decrease the survival of sympathetic neurons in the presence of NGF. MTT survival assays of sympathetic neurons selected in NGF, infected with 100–400 MOI of N-myc, GFP, or hypophosphorylated pRb adenovirus, maintained in NGF, and assayed 4 d after infection are shown. All conditions were performed in triplicate, and error bars represent the SD of the mean. Values derive from one representative experiment of three and are normalized to those obtained for uninfected neurons maintained in 10 ng/ml NGF, which are considered to be 100% survival. No significant alterations are seen in any of the conditions.B_–_D, Adenovirus-mediated expression of N-myc rescues sympathetic neurons from apoptosis induced by NGF withdrawal, as monitored by MTT assays (B), nuclear morphology (C), and Trypan blue exclusion (D). In all experiments, neurons were infected with adenovirus, withdrawn from NGF 2 d later, and analyzed after an additional 2 d. B, MTT assays of neurons infected with 80–320 MOI N-myc, GFP, or hypophosphorylated pRb adenovirus. Values were normalized to those obtained for uninfected neurons either maintained in NGF (100%) or withdrawn from NGF (1%). Values represent the average of three independent experiments, each of which was performed in triplicate. *p < 0.05; Student's t test comparing results obtained with the N-myc versus control GFP adenovirus. C,D, Percentage of living cells or nonapoptotic cells counted by Hoechst-labeled intact nuclei (C) or Trypan blue exclusion (D) after virus infection and NGF withdrawal. For both methods, values were obtained by counting three randomly selected microscope fields in each condition, and the error bars represent the SD of the mean. Values are representative results of one of two independent experiments. **p< 0.01; Student's t test comparing results obtained using the N-myc versus control β-galactosidase (LacZ) adenovirus.
Fig. 4.
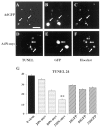
N-myc overexpression inhibits sympathetic neuron apoptosis after NGF withdrawal. A_–_F, TUNEL of sympathetic neurons infected with adenoviruses expressing GFP (AdGFP; A_–_C) or N-myc (this virus also expresses GFP) (AdN-myc;D_–_F) and withdrawn from NGF for 48 hr. Photomicrographs show TUNEL-positive nuclei (A, D), GFP-expressing cells (B, E), and Hoechst-positive nuclei (C, F) in the same fields (A_–_C and_D_–F). _Arrows_indicate the same neurons in each field. Note that neurons infected with the adenovirus expressing only GFP (A_–_C) are TUNEL positive and display shrunken, apoptotic nuclei, but that those infected with the virus expressing both GFP and N-myc (D_–_F) are not TUNEL positive. Scale bar, 100 μm. G, Percentage of TUNEL-positive cells 24 hr after NGF withdrawal. Neurons were infected with various MOIs of the N-myc or GFP adenoviruses, and the total number of TUNEL-positive nuclei in three randomly selected fields was counted. The values represent the average of two independent experiments, and error bars represent the SD of the mean. **p < 0.01 in Student's t test comparing apoptotic (TUNEL-positive) cell percentage after infection with AdN-myc versus AdGFP.
Fig. 5.
Constitutively hypophosphorylated pRb inhibits the S-phase entry, but not the survival, caused by N-myc.A, Constitutively hypophosphorylated pRb rescues the N-myc-induced BrdU incorporation. Neurons were coinfected with 50 MOI N-myc adenovirus and 50–200 MOI pRb adenovirus, incubated with BrdU 1 d later, and then analyzed immunocytochemically for BrdU and neurofilament-M after an additional 2 d. As a control, neurons were coinfected with 200 MOI of a β-galactosidase adenovirus (LacZ). Quantitation was performed as described in Figure 2_D. *p < 0.05; Student's_t test, comparing N-myc plus pRb versus N-myc plus β-galactosidase. B, pRb has no effect on N-myc-induced survival after NGF withdrawal. Neurons were infected with 200 MOI N-myc in the presence or absence of 100 or 200 MOI hypophosphorylated pRb adenovirus, and 2 d later NGF was withdrawn. Neurons were stained with Hoechst, and intact, nonapoptotic nuclei were quantitated by counting at least 300 cells in each of three random fields.C, AdN-myc decreases p75NTR expression and inhibits the activation of JNK. Immunoblots of sympathetic neurons infected with 100 or 400 MOI of N-myc adenovirus in the presence (right panel) or absence (left panel) of NGF. Left panel, Neurons were infected with N-myc adenovirus for 48 hr and then withdrawn from NGF for an additional 24 hr. Equal amounts of protein were analyzed for expression of the p75NTR (α-p75), the activated, phosphorylated form of JNK (α-P-JNK), or for total MAP kinase protein levels (α-MAPK). Note that although p75NTR and phospho-JNK levels were reduced on infection with N-myc, total MAP kinase levels remained the same. Right panel, Neurons were infected with N-myc adenovirus and maintained in NGF for 72 hr before Western blot analysis. Immunoblots were first probed with an antibody to p75NTR and then reprobed with an antibody for total α-tubulin to demonstrate that similar amounts of protein were present in each lane. Note the concentration-dependent decrease in p75NTR levels in neurons infected with the N-myc adenovirus. Similar results were obtained in four independent experiments.
Fig. 6.
N-myc does not cause BrdU incorporation in postmitotic cortical neurons. A_–_C, Immunocytochemical analysis of postmitotic cortical neuron cultures. Neurons were cultured as described in Results and double labeled with antibodies for neuron-specific MAP2 (A) and astrocyte-specific GFAP (B). Cells were also stained with Hoechst to show cell nuclei (C). The same field is shown in all three panels, and arrows_indicate the occasional astrocyte present in the cultures. Note that most fields did not contain any GFAP-positive cells.D_–_F, Cortical neurons were infected with 100 MOI N-myc adenovirus (which also expresses GFP) and then immunostained for MAP2. Visualization of GFP (D) revealed those cells that were transduced with the N-myc adenovirus, whereas immunostaining for MAP2 (E) indicated that the infected cells were neurons. F is the merged image resulting from D and E.Arrows indicate cells that were positive for both GFP and MAP2. G_–_I, Cortical neurons were infected with 100 MOI N-myc adenovirus and then incubated in the presence of BrdU for 24 hr before double-label immunocytochemical analysis for MAP2 (G) and BrdU (H). The cells were also stained with Hoechst (I). The same field is shown in all three panels, and the arrow indicates a non-MAP2-positive non-neuronal cell that is BrdU positive.J, Left two bars, Quantitation of data showing that N-myc fails to promote S-phase entry in postmitotic cortical neurons. Quantitation is of data similar to those shown in_D_–_F and indicates the percentage of MAP2-positive neurons that were GFP positive after infection with 100 MOI of N-Myc/GFP (100N-myc) or control GFP (100GFP) adenovirus. Right two bars, The percentage of MAP2-positive cells that were BrdU positive after infection with 100 MOI of either the N-myc or GFP adenoviruses. Similar results were obtained in three independent experiments.
Similar articles
- Proliferation and Survival of Embryonic Sympathetic Neuroblasts by MYCN and Activated ALK Signaling.
Kramer M, Ribeiro D, Arsenian-Henriksson M, Deller T, Rohrer H. Kramer M, et al. J Neurosci. 2016 Oct 5;36(40):10425-10439. doi: 10.1523/JNEUROSCI.0183-16.2016. J Neurosci. 2016. PMID: 27707976 Free PMC article. - Evidence that helix-loop-helix proteins collaborate with retinoblastoma tumor suppressor protein to regulate cortical neurogenesis.
Toma JG, El-Bizri H, Barnabe-Heider F, Aloyz R, Miller FD. Toma JG, et al. J Neurosci. 2000 Oct 15;20(20):7648-56. doi: 10.1523/JNEUROSCI.20-20-07648.2000. J Neurosci. 2000. PMID: 11027225 Free PMC article. - Regulation and deregulation of E2F1 in postmitotic neurons differentiated from embryonal carcinoma P19 cells.
Azuma-Hara M, Taniura H, Uetsuki T, Niinobe M, Yoshikawa K. Azuma-Hara M, et al. Exp Cell Res. 1999 Sep 15;251(2):442-51. doi: 10.1006/excr.1999.4593. Exp Cell Res. 1999. PMID: 10471329 - E2F1 and c-Myc in cell growth and death.
Matsumura I, Tanaka H, Kanakura Y. Matsumura I, et al. Cell Cycle. 2003 Jul-Aug;2(4):333-8. Cell Cycle. 2003. PMID: 12851485 Review. - Cell cycle regulators in neural stem cells and postmitotic neurons.
Yoshikawa K. Yoshikawa K. Neurosci Res. 2000 May;37(1):1-14. doi: 10.1016/s0168-0102(00)00101-2. Neurosci Res. 2000. PMID: 10802339 Review.
Cited by
- Interplay: The Essential Role between INSM1 and N-Myc in Aggressive Neuroblastoma.
Chen C, Lan MS. Chen C, et al. Biology (Basel). 2022 Sep 20;11(10):1376. doi: 10.3390/biology11101376. Biology (Basel). 2022. PMID: 36290282 Free PMC article. Review. - Pharmacophore-Model-Based Virtual-Screening Approaches Identified Novel Natural Molecular Candidates for Treating Human Neuroblastoma.
Opo FADM, Alkarim S, Alrefaei GI, Molla MHR, Alsubhi NH, Alzahrani F, Ahammad F. Opo FADM, et al. Curr Issues Mol Biol. 2022 Oct 13;44(10):4838-4858. doi: 10.3390/cimb44100329. Curr Issues Mol Biol. 2022. PMID: 36286044 Free PMC article. - LncRNA EPIC1 downregulation mediates hydrogen peroxide-induced neuronal cell injury.
Sun J, Zheng J, Li Y, Yan M, Li P, Ma L. Sun J, et al. Aging (Albany NY). 2019 Dec 8;11(23):11463-11473. doi: 10.18632/aging.102545. Epub 2019 Dec 8. Aging (Albany NY). 2019. PMID: 31812951 Free PMC article. - Neuroblastoma-A Neural Crest Derived Embryonal Malignancy.
Johnsen JI, Dyberg C, Wickström M. Johnsen JI, et al. Front Mol Neurosci. 2019 Jan 29;12:9. doi: 10.3389/fnmol.2019.00009. eCollection 2019. Front Mol Neurosci. 2019. PMID: 30760980 Free PMC article. Review. - Neuroblastoma Origin and Therapeutic Targets for Immunotherapy.
Kholodenko IV, Kalinovsky DV, Doronin II, Deyev SM, Kholodenko RV. Kholodenko IV, et al. J Immunol Res. 2018 Jul 11;2018:7394268. doi: 10.1155/2018/7394268. eCollection 2018. J Immunol Res. 2018. PMID: 30116755 Free PMC article. Review.
References
- Barrett GL, Georgious A, Reid K, Bartlett PF, Leung D. Rescue of dorsal root sensory neurons by nerve growth factor and neurotrophin-3, but not brain-derived neurotrophic factor or neurotrophin-4, is dependent on the level of the p75 neurotrophin receptor. Neuroscience. 1998;85:1321–1328. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous