Electrical synapses in the thalamic reticular nucleus - PubMed (original) (raw)
Electrical synapses in the thalamic reticular nucleus
Carole E Landisman et al. J Neurosci. 2002.
Abstract
Neurons of the thalamic reticular nucleus (TRN) provide inhibitory input to thalamic relay cells and generate synchronized activity during sleep and seizures. It is widely assumed that TRN cells interact only via chemical synaptic connections. However, we show that many neighboring pairs of TRN neurons in rats and mice are electrically coupled. In paired-cell recordings, electrical synapses were able to mediate close correlations between action potentials when the coupling was strong; they could modulate burst-firing states even when the coupling strength was more modest. Electrical synapses between TRN neurons were absent in mice with a null mutation for the connexin36 (Cx36) gene. Surprisingly, inhibitory chemical synaptic connections between pairs of neurons were not observed, although strong extracellular stimuli could evoke inhibition in single TRN neurons. We conclude that Cx36-dependent gap junctions play an important role in the regulation of neural firing patterns within the TRN. When combined with recent observations from the cerebral cortex, our results imply that electrical synapses are a common mechanism for generating synchrony within networks of inhibitory neurons in the mammalian forebrain.
Figures
Fig. 1.
Structure of the TRN slice and its neurons.A, Low-power IR-DIC image of the thalamic region of a living slice. Str, Striatum; IC, internal capsule. B, Higher-power view of a pair of TRN cells with adjoining somata (left). This pair was not electrically coupled. The right shows a pair of TRN cells with nonadjoining somata. These cells were strongly coupled (coupling coefficient of 0.1). Thus, the somata of cell pairs need not touch to be electrically coupled, and many pairs that do have adjacent somata are not coupled. C, Reconstruction of a neurobiotin-filled TRN neuron. Three major axonal branches leave the nucleus.
Fig. 2.
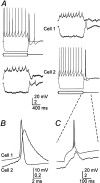
Electrical coupling between rat TRN neurons.A, Intracellular current steps (±200 pA) to evoke responses in cell 1 induced attenuated voltage changes in cell 2 (left traces) (coupling coefficient of 0.1). Tracings on the right are from the same cell pair, but, in this case, current steps were delivered to cell 2. B, Close-up of a single presynaptic spike and the averaged spikelet it induced in the soma of a coupled neighboring cell. C, Close-up of the rebound burst generated by cell 2 in A(right) and the coupling potential corresponding to it in cell 1.
Fig. 3.
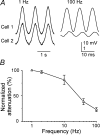
Frequency dependence of signal transfer between coupled neurons. A, Subthreshold sinusoidal currents were injected into one cell of a coupled pair, and membrane voltage deflections were recorded from the injected cell (top traces) and the cell coupled to it (bottom traces). Stimulus frequencies were 1 Hz (left traces) and 100 Hz (right traces). Note the differences in voltage gain between the traces of cell 1 (low gain) and cell 2 (high gain). B, Attenuation of sinusoidal signals across electrical synapses as a function of signal frequency. Data were obtained as described in A, and attenuation was defined as the ratio of peak-to-peak amplitudes in the noninjected compared with the injected cell, normalized to the 1 Hz attenuation and expressed as a percentage. Data points are the means ± SEM of measurements from four neuron pairs.
Fig. 4.
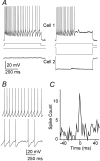
Electrical synapses can regulate spiking.A, Simultaneous recordings from a coupled TRN cell pair.Traces on the left show repetitive firing in cell 1 as it received a 100 pA pulse of depolarizing current; cell 2 received no current. Traces on the _right_show how a hyperpolarizing current step of −300 pA delivered to cell 2 reduced the duration and frequency of spiking induced by the same 100 pA stimulus to cell 1. B, Simultaneous current steps applied to a coupled pair (coupling coefficient of 0.1) evoked repetitive firing in both. Note the small spikelets in cell 2 during intervals of spike silence; each spikelet coincided with a spike in cell 1. C, Cross-correlogram of the spiking in the pair shown in B shows a sharp, narrow peak centered on 0 msec.
Fig. 5.
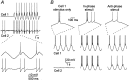
Modulation of spiking patterns mediated by electrical synapses. A, A sine wave current stimulus (1 Hz) was applied to cell 1, with the amplitude adjusted to generate one spike per cycle. Cell 2, which was electrically coupled to cell 1, was depolarized with steady current to near threshold. Sine wave stimuli to cell 1 induced entrained spikes in cell 2. The _traces_below show expansion of the traces above. Spikes in these panels are truncated. B, In a different pair of electrically coupled cells (coupling coefficient of 0.04), a 1 Hz sine wave stimulus to cell 1 induced firing of a single burst of spikes on each cycle (left). When both cells were stimulated with similar currents in-phase, cell 1 generated a burst plus two tonic spikes on each cycle (middle). When the same stimuli were shifted to anti-phase (right), cell 1 fired only two tonic spikes per cycle, and cell 2 fired a single spike per cycle. The top row shows expanded regions of recordings from cell 1, as indicated. Spike amplitudes have been truncated in all traces.
Fig. 6.
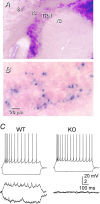
Electrical synapses in the TRN depend on Cx36.A, β-gal histochemistry of a section from a Cx36 KO mouse shows strong staining in the TRN but very weak staining in VB. The section was cut in the oblique plane used for electrophysiology (see Materials and Methods), and the reaction was strongly developed in an attempt to reveal staining in VB. B, Higher-power view of β-gal-reacted TRN, showing that some neurons stain strongly, whereas others are apparently unstained. In this case, the section was cut in the coronal plane, and the reaction time was optimized for the TRN. C, Moderate electrical coupling between a representative pair of TRN cells from a WT mouse (left); coupling was absent from almost all pairs of TRN cells from KO mice (right).
Fig. 7.
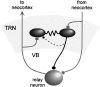
Synaptic circuitry of the TRN. Rectangular terminals, Excitatory chemical synapses; circular terminals, inhibitory chemical synapses; zigzagged lines, electrical synapses. The _dashed line_representing the intra-TRN chemical synaptic connection indicates uncertainty about its structural basis.
Similar articles
- A mixed electrical and chemical synapse in the thalamic reticular nucleus.
Landisman CE, Coulon P. Landisman CE, et al. J Neurophysiol. 2024 Dec 1;132(6):1955-1963. doi: 10.1152/jn.00339.2024. Epub 2024 Oct 30. J Neurophysiol. 2024. PMID: 39475494 - Electrical synapses and the development of inhibitory circuits in the thalamus.
Zolnik TA, Connors BW. Zolnik TA, et al. J Physiol. 2016 May 15;594(10):2579-92. doi: 10.1113/JP271880. Epub 2016 Mar 23. J Physiol. 2016. PMID: 26864476 Free PMC article. - Small clusters of electrically coupled neurons generate synchronous rhythms in the thalamic reticular nucleus.
Long MA, Landisman CE, Connors BW. Long MA, et al. J Neurosci. 2004 Jan 14;24(2):341-9. doi: 10.1523/JNEUROSCI.3358-03.2004. J Neurosci. 2004. PMID: 14724232 Free PMC article. - Connexon connexions in the thalamocortical system.
Cruikshank SJ, Landisman CE, Mancilla JG, Connors BW. Cruikshank SJ, et al. Prog Brain Res. 2005;149:41-57. doi: 10.1016/S0079-6123(05)49004-4. Prog Brain Res. 2005. PMID: 16226575 Review. - Electrical synapses in the mammalian brain.
Connors BW, Long MA. Connors BW, et al. Annu Rev Neurosci. 2004;27:393-418. doi: 10.1146/annurev.neuro.26.041002.131128. Annu Rev Neurosci. 2004. PMID: 15217338 Review.
Cited by
- Regulatory Roles of Metabotropic Glutamate Receptors on Synaptic Communication Mediated by Gap Junctions.
Cachope R, Pereda AE. Cachope R, et al. Neuroscience. 2021 Feb 21;456:85-94. doi: 10.1016/j.neuroscience.2020.06.034. Epub 2020 Jun 30. Neuroscience. 2021. PMID: 32619474 Free PMC article. Review. - Characteristics and plasticity of electrical synaptic transmission.
Curti S, O'Brien J. Curti S, et al. BMC Cell Biol. 2016 May 24;17 Suppl 1(Suppl 1):13. doi: 10.1186/s12860-016-0091-y. BMC Cell Biol. 2016. PMID: 27230893 Free PMC article. Review. - Sensing and processing whisker deflections in rodents.
Burns TF, Rajan R. Burns TF, et al. PeerJ. 2021 Feb 22;9:e10730. doi: 10.7717/peerj.10730. eCollection 2021. PeerJ. 2021. PMID: 33665005 Free PMC article. - Computational model of electrically coupled, intrinsically distinct pacemaker neurons.
Soto-Treviño C, Rabbah P, Marder E, Nadim F. Soto-Treviño C, et al. J Neurophysiol. 2005 Jul;94(1):590-604. doi: 10.1152/jn.00013.2005. Epub 2005 Feb 23. J Neurophysiol. 2005. PMID: 15728775 Free PMC article. - Downregulation of Neuronal and Dendritic Connexin36-Made Electrical Synapses Without Glutamatergic Axon Terminals in Spinal Anterior Horn Cells From the Early Stage of Amyotrophic Lateral Sclerosis.
Kobayakawa Y, Masaki K, Yamasaki R, Shiraishi W, Hayashida S, Hayashi S, Okamoto K, Matsushita T, Kira JI. Kobayakawa Y, et al. Front Neurosci. 2018 Nov 28;12:894. doi: 10.3389/fnins.2018.00894. eCollection 2018. Front Neurosci. 2018. PMID: 30546295 Free PMC article.
References
- Avanzini G, Panzica F, de Curtis M. The role of the thalamus in vigilance and epileptogenic mechanisms. Clin Neurophysiol. 2000;111 [Suppl 2]:S19–S26. - PubMed
- Bazhenov M, Timofeev I, Steriade M, Sejnowski TJ. Self-sustained rhythmic activity in the thalamic reticular nucleus mediated by depolarizing GABAA receptor potentials. Nat Neurosci. 1999;2:168–174. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- NS25983/NS/NINDS NIH HHS/United States
- R01 NS025983/NS/NINDS NIH HHS/United States
- R01 GM018974/GM/NIGMS NIH HHS/United States
- GM37751/GM/NIGMS NIH HHS/United States
- GM18974/GM/NIGMS NIH HHS/United States
- Wellcome Trust/United Kingdom
- R01 GM037751/GM/NIGMS NIH HHS/United States
- NS27248/NS/NINDS NIH HHS/United States
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous