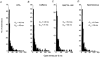A Ca2+-permeable non-selective cation channel activated by depletion of internal Ca2+ stores in single rabbit portal vein myocytes - PubMed (original) (raw)
A Ca2+-permeable non-selective cation channel activated by depletion of internal Ca2+ stores in single rabbit portal vein myocytes
A P Albert et al. J Physiol. 2002.
Abstract
In vascular smooth muscle cells many agonists cause the release of Ca2+ ions from internal stores. An important problem concerns the mechanism by which the intracellular stores are refilled subsequent to depletion. In the present study, we describe the properties of a Ca2+-permeable non-selective cation channel current that is activated in rabbit portal vein myocytes by depletion of internal Ca2+ stores. Application of cyclopiazonic acid (CPA), which depletes internal Ca2+ stores, activated whole-cell currents that had a reversal potential (E(r)) of about +50 mV in 1.5 mM external Ca2+ (Ca2+o). In 0 mM Ca2+o, the currents were larger and E(r) was approximately 0 mV. Application of CPA and caffeine during cell-attached recording activated single inward channel currents at negative potentials, which had a slope conductance of 2-3 pS and an E(r) of +20 mV. The slope conductance in 0 and 110 mM Ca2+o was 7 and 1.5 pS, respectively, and E(r) values indicated that these non-selective cation channels are highly permeable to Ca2+ ions. Bath application of the cell-permeant Ca2+ chelator, BAPTA-AM, also activated similar currents, indicating that these channels are not activated by Ca2+. Spontaneous channel currents with similar properties to store-operated channels were observed in some patches. Application of W-7, an inhibitor of the Ca2+-binding protein calmodulin, also activated similar Ca2+-permeable channel currents. In conclusion, it is demonstrated that agents that deplete Ca2+ stores and inhibit calmodulin binding activate Ca2+-permeable non-selective cation channel currents in rabbit portal vein myocytes. These channels may have an important role in vascular smooth muscle in providing an influx of Ca2+ to refill depleted internal Ca2+ stores and appear to possess different characteristics to store-operated channels described in other vascular smooth muscle preparations.
Figures
Figure 1. CPA activates a non-selective cation current in rabbit portal vein smooth muscle cells recorded with the whole-cell method
A, the mean time course of the CPA-evoked cation currents recorded at −50 mV in either 1.5 m
m
(•) or 0 m
m
(○) Cao2+. Each data point is the mean of at least 4 cells. B, examples of I_–_V relationship of the CPA-evoked cation currents recorded in either 1.5 m
m
or 0 Cao2+. Note the larger CPA-evoked current, linear I_–_V relationship and more negative reversal potential (_E_r) in 0 m
m
Cao2+ compared to 1.5 m
m
Cao2+. C, the mean I-V relationships of the CPA-evoked cation currents in 1.5 m
m
(•) or 0 m
m
(○) Cao2+. Each point is the mean of 5 cells. The plots have been normalized to the amplitudes of the whole-cell cation currents in 1.5 m
m
Cao2+ at −50 mV (= −1 on _y_-axis).
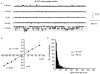
Figure 2. Application of CPA or caffeine activates single channel currents in cell-attached patches from rabbit portal vein smooth muscle cells
A, using 126 m
m
NaCl with 1.5 m
m
Ca2+ patch pipette solution, bath application of 10 μ
m
CPA activated single channel currents in a patch that had not previously shown single channel activity. Note that inward currents are represented as downward deflections and that there is no CPA-evoked single channel activity at +100 mV. Continuous lines indicate the closed level, whereas dashed lines indicate open levels. B, the I_–_V relationship of the CPA-evoked single inward channel currents shown in A had a slope conductance (γ) of 2.1 pS between −120 and −40 mV and an extrapolated _E_r (↓) of +23 mV. C, bath application of 10 m
m
caffeine evoked single channel currents in a different cell-attached patch. Note the multiple single channel current openings at negative patch potentials. D, the I_–_V relationship of the caffeine-evoked single inward currents shown in C that had a slope conductance of 2 pS between −120 and −40 mV and an extrapolated _E_r (↓) of +22 mV.
Figure 3. Application of the cell-permeant Ca2+ chelator, BAPTA-AM, activates inward channel currents that have similar properties to the CPA- and caffeine-evoked channel currents
A, activation of inward channel currents at negative membrane potentials after bath application of BAPTA-AM for ∼20 min. Note that no channel currents could be observed at positive membrane potentials. B, the I_–_V relationship of the channel currents shown in A. The channel currents had a slope conductance of 2.3 pS between −120 and −40 mV and an extrapolated _E_r (↓) of +25 mV.
Figure 4. Application of CPA activates single cation channels that are permeable to Ca2+ ions
A, with a 110 m
m
CaCl2 patch pipette solution, bath application of 10 μ
m
CPA activated single cation currents in a previously quiescent patch. The CPA-evoked single cation currents were only observed at negative patch potentials. B, the pooled I_–_V relationships of CPA-evoked single cation currents recorded with 110 m
m
CaCl2 patch pipette solution. The I_–_V relationship had a slope conductance of 1.5 pS between −120 and −40 mV and an extrapolated _E_r (↓)of +86 mV. Each point is the mean of at least 4 patches.
Figure 5. Cell-attached patches contain spontaneous single channel currents with characteristics similar to the CPA- and caffeine-evoked cation channels
A, spontaneous channel activity between −40 and −120 mV in one patch recorded with 126 m
m
NaCl patch pipette solution. B, pooled I_–_V relationship of spontaneous non-selective cation channels recorded with 126 m
m
NaCl with 1.5 m
m
Cao2+ (•) or 110 m
m
CaCl2 (○) in the patch pipette solution. In 126 m
m
NaCl, the I_–_V relationship had a slope conductance of 2 pS between −120 and −40 mV and an extrapolated _E_r (↓) of +18 mV. In 110 m
m
CaCl2, the I_–_V relationship had a slope conductance of 1.3 pS and an extrapolated _E_r (↓) of + 98 mV. Each point is the mean of at least 4 patches.
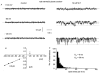
Figure 6. Open lifetime distributions of the CPA-, caffeine-, BAPTA-AM-evoked and spontaneous cation channel currents recorded with a 126 mm NaCl patch pipette solution
The open lifetime distributions of CPA- (A), caffeine- (B), BAPTA-AM-evoked (C) and spontaneous (D) cation channels could all be fitted with (continuous lines) the sum of two exponentials with time constants of approximately 5 ms (Oτ1) and 30 ms (Oτ2). The holding potential was −80 mV in all cases.
Figure 7. Characteristics of non-selective cation channel currents in the absence of Cao2+
A, spontaneous channel currents recorded at different membrane potentials with a 0 m
m
Cao2+ patch pipette solution. Note that outward channel currents (denoted by upward deflections) can be observed at positive membrane potentials. B, the I_–_V relationship of the channel currents shown in A. The I_–_V relationship was linear between −110 and +80 mV with a slope conductance of 8 pS and an _E_r of −4 mV. C, open time distribution of the channel currents shown in A at −110 mV. The open times could be described by the sum of two exponentials with time constants of 5.4 ms (Oτ1) and 32 ms (Oτ2).
Figure 8. Application of the calmodulin inhibitor, W-7, activates single channel currents with properties that are similar to CPA-evoked cation channel currents
A, with a 126 m
m
NaCl pipette solution, bath application of 50 μ
m
W-7 evoked single channel currents at negative patch potentials. B, the I_–_V relationship of the W-7-evoked single channels currents shown in A. C, at −80 mV, the open time distribution of the W-7-evoked single channels shown in A could be described by the sum of two exponentials with time constants of 5 ms (Oτ1) and 36 ms (Oτ2).
Similar articles
- Properties of a native cation channel activated by Ca2+ store depletion in vascular smooth muscle cells.
Trepakova ES, Gericke M, Hirakawa Y, Weisbrod RM, Cohen RA, Bolotina VM. Trepakova ES, et al. J Biol Chem. 2001 Mar 16;276(11):7782-90. doi: 10.1074/jbc.M010104200. Epub 2000 Dec 11. J Biol Chem. 2001. PMID: 11113149 - The effect of external divalent cations on spontaneous non-selective cation channel currents in rabbit portal vein myocytes.
Albert AP, Large WA. Albert AP, et al. J Physiol. 2001 Oct 15;536(Pt 2):409-20. doi: 10.1111/j.1469-7793.2001.0409c.xd. J Physiol. 2001. PMID: 11600676 Free PMC article. - Large conductance calcium-activated non-selective cation channel in smooth muscle cells isolated from rat portal vein.
Loirand G, Pacaud P, Baron A, Mironneau C, Mironneau J. Loirand G, et al. J Physiol. 1991 Jun;437:461-75. doi: 10.1113/jphysiol.1991.sp018606. J Physiol. 1991. PMID: 1716314 Free PMC article. - Store-operated Ca2+-permeable non-selective cation channels in smooth muscle cells.
Albert AP, Large WA. Albert AP, et al. Cell Calcium. 2003 May-Jun;33(5-6):345-56. doi: 10.1016/s0143-4160(03)00048-4. Cell Calcium. 2003. PMID: 12765681 Review. - Receptor-operated Ca2(+)-permeable nonselective cation channels in vascular smooth muscle: a physiologic perspective.
Large WA. Large WA. J Cardiovasc Electrophysiol. 2002 May;13(5):493-501. doi: 10.1046/j.1540-8167.2002.00493.x. J Cardiovasc Electrophysiol. 2002. PMID: 12030534 Review.
Cited by
- Physiological mechanisms of TRPC activation.
Putney JW. Putney JW. Pflugers Arch. 2005 Oct;451(1):29-34. doi: 10.1007/s00424-005-1416-4. Epub 2005 Aug 18. Pflugers Arch. 2005. PMID: 16133266 Review. - Discrete store-operated calcium influx into an intracellular compartment in rabbit arteriolar smooth muscle.
Flemming R, Cheong A, Dedman AM, Beech DJ. Flemming R, et al. J Physiol. 2002 Sep 1;543(Pt 2):455-64. doi: 10.1113/jphysiol.2002.023366. J Physiol. 2002. PMID: 12205181 Free PMC article. - Activation of store-operated channels by noradrenaline via protein kinase C in rabbit portal vein myocytes.
Albert AP, Large WA. Albert AP, et al. J Physiol. 2002 Oct 1;544(Pt 1):113-25. doi: 10.1113/jphysiol.2002.022574. J Physiol. 2002. PMID: 12356885 Free PMC article. - Dual effect of calmodulin on store-operated Ca2+ -permeable cation channels in rabbit portal vein myocytes.
Albert AP, Liu M, Large WA. Albert AP, et al. Br J Pharmacol. 2006 Aug;148(7):1001-11. doi: 10.1038/sj.bjp.0706797. Epub 2006 Jun 12. Br J Pharmacol. 2006. PMID: 16770321 Free PMC article. - Sarcoplasmic reticulum Ca2+ release and depletion fail to affect sarcolemmal ion channel activity in mouse skeletal muscle.
Allard B, Couchoux H, Pouvreau S, Jacquemond V. Allard B, et al. J Physiol. 2006 Aug 15;575(Pt 1):69-81. doi: 10.1113/jphysiol.2006.112367. Epub 2006 Jun 15. J Physiol. 2006. PMID: 16777939 Free PMC article.
References
- Golovina VA, Platoshyn O, Bailey CL, Wang J, Limsuwan A, Sweeney M, Rubin LJ, Yuan JX-J. Upregulated TRP and enhanced capacitative Ca2+ entry in human pulmonary artery myocytes during proliferation. American Journal of Physiology - Heart and Circulatory Physiology. 2001;H280:746–755. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous