Drosophila Aurora A kinase is required to localize D-TACC to centrosomes and to regulate astral microtubules - PubMed (original) (raw)
Drosophila Aurora A kinase is required to localize D-TACC to centrosomes and to regulate astral microtubules
Régis Giet et al. J Cell Biol. 2002.
Abstract
Disruption of the function of the A-type Aurora kinase of Drosophila by mutation or RNAi leads to a reduction in the length of astral microtubules in syncytial embryos, larval neuroblasts, and cultured S2 cells. In neuroblasts, it can also lead to loss of an organized centrosome and its associated aster from one of the spindle poles, whereas the centrosome at the other pole has multiple centrioles. When centrosomes are present at the poles of aurA mutants or aurA RNAi spindles, they retain many antigens but are missing the Drosophila counterpart of mammalian transforming acidic coiled coil (TACC) proteins, D-TACC. We show that a subpopulation of the total Aurora A is present in a complex with D-TACC, which is a substrate for the kinase. We propose that one of the functions of Aurora A kinase is to direct centrosomal organization such that D-TACC complexed to the MSPS/XMAP215 microtubule-associated protein may be recruited, and thus modulate the behavior of astral microtubules.
Figures
Figure 1.
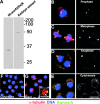
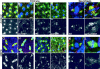
Aurora A localizes to the centrosomes and the spindle poles independently of microtubules. (A) An anti–Aurora A anti-serum recognizes the NH2-terminal recombinant histidine-tagged protein domain used for immunization (left) and the 47-kD endogenous Aurora A protein kinase in Drosophila embryo extracts (right) by Western blotting. Similar results were obtained using affinity purified antibodies. (B–E) The Aurora A antibody (green) decorates centrosomes and spindle poles in S2 cultured cells in prophase (B), metaphase (C), anaphase (D), and cytokinesis (E). Microtubules are in red, DNA in blue. Right hand panels show Aurora A staining alone. Note the decrease in the Aurora A staining during cytokinesis (E, right, arrow). Bar is 10 μm. Colchicine (F) and taxol (G) treatments have no effects on the Aurora A centrosome localization. Note that aurora remains on the asters containing the centrosome (G, inset). Microtubules are red, aurora is green and DNA is blue and the scale bar represents 10 μm. Inset, α-tubulin (red); bar, 5 μm.
Figure 3.
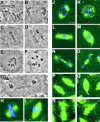
Mutation in aurora A leads to metaphase arrest in neuroblasts and decreased length of astral microtubules in both neuroblasts and embryos. Squashed preparations of larval brains from wild-type (A and B) or aurA e209/Df(3R)T61 (C–G) were stained with aceto-orcein and the mitotic figures were scored. A and B wild-type metaphase and anaphase figures, respectively. (C and D) aurA e209Df(3R)T61 metaphase-like figures spindles and E and F circular mitotic figures. Note the high chromosome condensation in panels C–F. (G) An abnormal anaphase figure with lagging chromosomes. (H–S) aurA mutants have short astral microtubules in mitosis. (H and I) Spindles from wild- type and aurA e209/Df(3R)T61 mutant neuroblasts, respectively. Arrow point to a small aster at the mutant spindle poles. Bar, 10 μm. Wild-type embryos (J, prophase; L, early anaphase; N, telophase) and aurA 287-derived embryos (K, prophase; M, early anaphase; O, telophase) that have been fixed and stained for tubulin (green) or DNA (blue). Note the decreased length of the astral microtubules in the mutant embryos (arrows). (P–S) Microtubule behavior monitored in live wild type (P, early anaphase; R, telophase) and aurA 287-derived embryos (Q, early anaphase; S, telophase) expressing a tau–GFP transgene under the control of the polyubiquitin promoter. Mutant embryos show a reduced number of apparently shorter astral microtubules (arrows). Bar, 5 μm.
Figure 2.
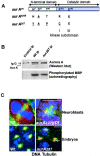
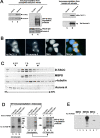
_aurA_287 encodes an enzyme of reduced activity that localizes to the centrosome. aurA e209 and aurA 287 encode full-length Aurora A kinases with the indicated amino acid changes (underlined). The amino acid changes in aurA e209 were previously reported (Glover et al., 1995). Such a mutation was described to be essential for kinase activity (Hunter and Sefton, 1981). (B) The aurA 287 mutant shows reduced protein kinase activity. Extracts of wild-type or aurA 287-derived embryos (lanes 2 and 3, respectively) were submitted to immunoprecipitation using anti–Aurora A antibodies. One aliquot of immunoprecipitate was subjected to Western blotting (top panels) and a second (bottom panels) assayed for its ability to phosphorylate myelin basic protein (Materials and methods). Quantification using a phosphoimager identified the mutant Aurora A287 kinase had 35% of the activity of wild-type. (C) Mutations in aurA do not prevent its centrosome localization. Wild-type (top left) or auroraA e209 (top right) neuroblasts showing localization of Aurora A (red) to centrosomes of a bipolar and monopolar spindle respectively. Embryos derived from wild-type (bottom left) or homozygous aurora A 287 mothers (bottom right) showing Aurora A (red) at centrosomes. The arrow shows a centrosome pair that has dissociated from the nucleus. DNA is stained blue and tubulin, green. Bars, 5 μM.
Figure 4.
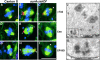
_aurA_e209/Df(3R)T61 neuroblasts show abnormal mitotic spindle poles. Localization of γ-tubulin (A–C), centrosomin (D–F) and CP190 (G–I) in wild-type Canton S (A, D, and G) or aurA e209/Df(3R)T61 metaphase spindles (B, C, E, F, H, and I). Note the absence of centrosomal antigens at the poles indicated by arrowheads (C and I) and the presence of additional bodies of staining (B, E, H, arrows). Cnn, γ-tubulin and CP190 are shown in red, microtubules in green and DNA in blue. Bar is 10 μm. (J and K) ultrastructure of an aurA e209/Df(3R)T61 spindle pole by electron microscopy (one section). Chromosomes are indicated Ch. Note the presence of 5 centrioles on the spindle pole, boxed and shown enlarged in K. Bars: (J) 5 μm; (K) 1 μm.
Figure 5.
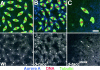
D-TACC and MSPS proteins are mislocalized in aurA mutant cells. D-TACC (A–F) and MSPS (G–L) localization in wild-type (A, prophase; C and I, telophase; G, metaphase) or AurA 287 mutant embryos (B, prophase; H, metaphase; D and J, telophase). Staining was also performed in wild-type (E and K) or aurA e209/Df neuroblasts (F and L). In all color panels, D-TACC and MSPS are red, microtubules are green and DNA is blue. Lower panels show D-TACC or MSPS staining alone in monochrome. Note that D-TACC and MSPS disappears from the poles of aurA mutant cells (arrows) but remains associated with spindle microtubules. In aurA e209 neuroblasts, both D-TACC and MSPS tends to generate aggregates in the cytoplasm or that stick on the mitotic spindles. Bars, 5 μm.
Figure 6.
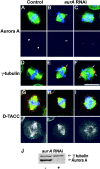
S2 cells display spindle pole abnormalities following aurA RNAi. Control (A, D, and G) and aurA RNAi treated (B, C, E, F, H, and I) S2 cells immunostained to reveal either Aurora A (A-C), gamma-tubulin (D–F), or D-TACC (G–I) in red. DNA is stained blue and microtubules, green. Monochrome images show the red channel alone and the scale bar represents 10 μm. Arrows in panel B indicate reduced levels of Aurora A staining and reduced length and density of astral microtubules following aurA RNAi. Arrows in panels E and F indicate multiple gamma-tubulin containing bodies at the poles and reduced length and density of astral microtubules in aurA RNAi cells. J, aurora A and γ-tubulin Western blot of S2 cells 3 d after aurA RNAi treatment. Aurora A levels were reduced by >95%, whereas Aurora B, or D-TACC protein levels were unchanged (unpublished data).
Figure 7.
Aurora A is found on centrosomes in d-tacc mutants. Embryos derived from wild-type (A) d-tacc 1, (B) or d-tacc stella, (C) mothers stained to reveal microtubules (green), DNA (red) and aurora A (blue). Bar, 10 μm.
Figure 8.
Aurora A interacts with and phosphorylates D-TACC. (A) Drosophila Aurora A (lane 2) and GFP–D-TACC coimmunoprecipitate (lane 4) and human (Hs) Aurora A precipitates with Hs TACC3 (lane 6). Lanes 1 and 2 show control preimmune and anti–Aurora A immunoprecipitates blotted to reveal D-TACC (top) or Aurora A (bottom). Lanes 3 and 4 show anti-GFP immunoprecipitates from transgenic lines expressing Tau–GFP and D-TACC–GFP fusion proteins, respectively and blotted with anti–D-TACC (top) or anti–Aurora A (bottom). Lanes 5 and 6 show control preimmune and anti-human Aurora A immunoprecipitates blotted to reveal human TACC3 (top) or human Aurora A (bottom). (B) Hs aurora (green) colocalizes with Hs TACC3 (red) to the centrosomes and the spindle poles. DNA is blue. (C) Extracts of a 2-h collection of Drosophila embryos were fractionated on a 5–40% sucrose gradient. Aliquots from each fraction were subjected to Western blotting with anti-TACC antibodies (top), anti-minispindle (second panel from the top), anti–γ-tubulin (second panel from the bottom) and anti–Aurora A (bottom). Molecular mass marker (4.3, 7.4, and 11 S are indicated). (D) GFP–D-TACC (lanes 2, 3, 5, 6, 8, and 9) or GFP-Tau (lanes 1, 4, and 7) were immunoprecipitated from Drosophila embryos, heat treated to inactivate potential endogenous kinases, and incubated with recombinant aurA-(His)6 kinase (lanes labeled +) and [γ-32P] ATP. Enzyme was not added to the reaction mixtures analyzed on lanes marked −. Left and middle panels show the radioactive products of the same kinase assay analyzed by 10 and 6% SDS-PAGE, respectively. The right panel shows an equivalent aliquot of material from a 6% gel analyzed by Western blotting with an anti-GFP antibody. Note the correspondence between the phosphorylated bands in the middle pannel and the presence of the GFP-D-TACC protein and its degradation products in the right panel. (E) Bacterially expressed chimeras of Maltose Binding Protein fused to the NH2-terminal (MBP–Nt amino acids 2–433), middle (MBP-Mid, amino acids 433–889), or COOH-terminal domain (MBP–Ct, amino acids 853–1189) of D-TACC were incubated in the presence (+, lanes 2, 4, and 6) or absence (−, lanes 1, 3, and 5) of purified aurora A-(His) 6 kinase and [γ-32P] ATP. Note the strong phosphorylation of the middle fragment of D-TACC (lane 4) and the very weak phosphorylation of the MBP–Nt and MBP–Ct fusion proteins (lanes 2 and 6).
Similar articles
- Aurora A activates D-TACC-Msps complexes exclusively at centrosomes to stabilize centrosomal microtubules.
Barros TP, Kinoshita K, Hyman AA, Raff JW. Barros TP, et al. J Cell Biol. 2005 Sep 26;170(7):1039-46. doi: 10.1083/jcb.200504097. J Cell Biol. 2005. PMID: 16186253 Free PMC article. - Dynamic association of a tumor amplified kinase, Aurora-A, with the centrosome and mitotic spindle.
Stenoien DL, Sen S, Mancini MA, Brinkley BR. Stenoien DL, et al. Cell Motil Cytoskeleton. 2003 Jun;55(2):134-46. doi: 10.1002/cm.10120. Cell Motil Cytoskeleton. 2003. PMID: 12740874 - Msps/XMAP215 interacts with the centrosomal protein D-TACC to regulate microtubule behaviour.
Lee MJ, Gergely F, Jeffers K, Peak-Chew SY, Raff JW. Lee MJ, et al. Nat Cell Biol. 2001 Jul;3(7):643-9. doi: 10.1038/35083033. Nat Cell Biol. 2001. PMID: 11433296 - Role of centrosomal adaptor proteins of the TACC family in the regulation of microtubule dynamics during mitotic cell division.
Thakur HC, Singh M, Nagel-Steger L, Prumbaum D, Fansa EK, Gremer L, Ezzahoini H, Abts A, Schmitt L, Raunser S, Ahmadian MR, Piekorz RP. Thakur HC, et al. Biol Chem. 2013 Nov;394(11):1411-23. doi: 10.1515/hsz-2013-0184. Biol Chem. 2013. PMID: 23787465 Review. - Centrosomes and cancer: lessons from a TACC.
Raff JW. Raff JW. Trends Cell Biol. 2002 May;12(5):222-5. doi: 10.1016/s0962-8924(02)02268-7. Trends Cell Biol. 2002. PMID: 12062169 Review.
Cited by
- Multiple Functions of the Essential Gene PpV in Drosophila Early Development.
Liu B, Sung HW, Großhans J. Liu B, et al. G3 (Bethesda). 2019 Nov 5;9(11):3583-3593. doi: 10.1534/g3.119.400662. G3 (Bethesda). 2019. PMID: 31484673 Free PMC article. - Insights into the non-mitotic functions of Aurora kinase A: more than just cell division.
Bertolin G, Tramier M. Bertolin G, et al. Cell Mol Life Sci. 2020 Mar;77(6):1031-1047. doi: 10.1007/s00018-019-03310-2. Epub 2019 Sep 27. Cell Mol Life Sci. 2020. PMID: 31562563 Free PMC article. Review. - Drosophila Aurora-A kinase inhibits neuroblast self-renewal by regulating aPKC/Numb cortical polarity and spindle orientation.
Lee CY, Andersen RO, Cabernard C, Manning L, Tran KD, Lanskey MJ, Bashirullah A, Doe CQ. Lee CY, et al. Genes Dev. 2006 Dec 15;20(24):3464-74. doi: 10.1101/gad.1489406. Genes Dev. 2006. PMID: 17182871 Free PMC article. - Phosphorylation and stabilization of HURP by Aurora-A: implication of HURP as a transforming target of Aurora-A.
Yu CT, Hsu JM, Lee YC, Tsou AP, Chou CK, Huang CY. Yu CT, et al. Mol Cell Biol. 2005 Jul;25(14):5789-800. doi: 10.1128/MCB.25.14.5789-5800.2005. Mol Cell Biol. 2005. PMID: 15987997 Free PMC article. - Aurora A contributes to p150(glued) phosphorylation and function during mitosis.
Romé P, Montembault E, Franck N, Pascal A, Glover DM, Giet R. Romé P, et al. J Cell Biol. 2010 May 17;189(4):651-9. doi: 10.1083/jcb.201001144. J Cell Biol. 2010. PMID: 20479466 Free PMC article.
References
- Adams, R.R., S.P. Wheatleya, A.M. Gouldsworthy, S.E. Kandels-Lewis, M. Carmena, C. Smythe, D.L. Gerloff, and W.C. Earnshaw. 2000. INCENP binds the aurora-related kinase AIRK2 and is required to target it to chromosomes, the central spindle and cleavage furrow. Curr. Biol. 10:1075–1078. - PubMed
- Andersen, S.S., A.J. Ashford, R. Tournebize, O. Gavet, A. Sobel, A.A. Hyman, and E. Karsenti. 1997. Mitotic chromatin regulates phosphorylation of Stathmin/Op18. Nature. 389:640–643. - PubMed
- Belmont, L., T. Mitchison, and H.W. Deacon. 1996. Catastrophic revelations about Op18/stathmin. Trends Biochem. Sci. 21:197–198. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous