Arteriolar and venular patterning in retinas of mice selectively expressing VEGF isoforms - PubMed (original) (raw)
. 2002 Feb;109(3):327-36.
doi: 10.1172/JCI14362.
Yin-Shan Ng, Richard Rohan, Marcus Fruttiger, Ann Bouché, Ali Yuce, Hajime Fujisawa, Bart Hermans, Moshe Shani, Sandra Jansen, Dan Hicklin, David J Anderson, Tom Gardiner, Hans-Peter Hammes, Lieve Moons, Mieke Dewerchin, Désiré Collen, Peter Carmeliet, Patricia A D'Amore
Affiliations
- PMID: 11827992
- PMCID: PMC150858
- DOI: 10.1172/JCI14362
Arteriolar and venular patterning in retinas of mice selectively expressing VEGF isoforms
Ingeborg Stalmans et al. J Clin Invest. 2002 Feb.
Abstract
The murine VEGF gene is alternatively transcribed to yield the VEGF(120), VEGF(164), and VEGF(188) isoforms, which differ in their potential to bind to heparan sulfate and neuropilin-1 and to stimulate endothelial growth. Here, their role in retinal vascular development was studied in mice selectively expressing single isoforms. VEGF(164/164) mice were normal, healthy, and had normal retinal angiogenesis. In contrast, VEGF(120/120) mice exhibited severe defects in vascular outgrowth and patterning, whereas VEGF(188/188) mice displayed normal venular outgrowth but impaired arterial development. It is noteworthy that neuropilin-1, a receptor for VEGF(164), was predominantly expressed in retinal arterioles. These findings reveal distinct roles of the various VEGF isoforms in vascular patterning and arterial development in the retina.
Figures
Figure 1
Targeting strategy. Modification of the VEGF gene to generate the VEGF164 targeting vector. Top: Targeting vector pPNT.VEGF164 and wild-type VEGF allele (VEGFWT). Middle: Homologously recombined VEGF164 allele (VEGF164neo). Bottom: Modified VEGF164 allele after Cre excision of the lox P–flanked neo cassette.
Figure 2
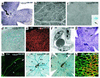
Retinal vascular development in VEGF+/+ and VEGF164/164 mice. (a) PECAM staining in VEGF+/+ at P5. Capillaries reach two-thirds to three-quarters of the retinal radius. Arterioles (A) are thin and have a capillary-free zone. Venules (V) are broader and lack a capillary-free zone. La, arteriolar length; Lv, venular length; Lr, radius of retina; Lva, radius of vascular bed. (b and c) In situ hybridization for ephrin-B2 (EB2) (b) with or (c) without collagen type IV fluorescent immunostaining on VEGF+/+ retina at P5 (EB2/CollIV). EB2 expression is confined to morphologically defined arterioles (see above) and extends into arterial capillaries (arrows). (d and e) Triple staining with LacZ (in PECs, blue dots), lectin (in ECs, red), and anti-SMA (in vSMCs, green) on a VEGF+/+ × PECLacZ P5 retina (LacZ/Lec/SMA). (d) PECs have spread along all retinal vessels, whereas (e) only arterioles are SMA positive. (f) EM of VEGF+/+ capillary at P5, which appears constricted. (g) PECAM staining on VEGF+/+ P9 retina. (h) SMA staining in VEGF+/+ at P14 is confined to arterioles and their side branches. (i) VEGF+/+ × PECLacZ retina at 4 weeks. Hyaloid arteries (HA) have regressed (HA remnant, arrow). (j) Double staining for lectin (red) and GFAP (in ACs, green) (Lec/GFAP). AC network in front of vasculature. (k) PECAM staining of VEGF164/164 retina at P5. Normal vascular development.
Figure 3
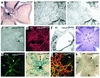
Retinal vascular development in VEGF188/188 mice. (a) PECAM staining at P5. Capillary outgrowth comparable to VEGF+/+ retinas, but no morphologically defined arterioles. (b and c) EB2/CollIV at P5. No EB2 expression at P5. Inset in c: VEGF188/188 × EB2LacZ retina at P6 shows small ephrin-B2–positive vessel (arrow). (d and e) LacZ/Lec/SMA on a VEGF188/188 × PECLacZ retina at P5, revealing (b) high PC density, (c) but no SMA immunoreactivity. (f) EM at P5 shows normal capillary constriction. (g) PECAM staining at P9 with increased capillary density in the midperipheral retina (arrow) and partially removed hyaloid arteries. (h) SMA immunoreactivity is attenuated in arterioles, but has become positive in venules and capillaries (arrow) at P14. (i and j) VEGF188/188 × PECLacZ retina at 4 weeks. (i) Persistence of hyaloid arteries and underdevelopment of arterioles. (j) Same retina after dissection of hyaloid arteries. Central part, but not peripheral part (arrows), of hyaloid arteries can be removed. (k) Lec/GFAP. ACs are organized into a tubular network well in front of the vascular bed.
Figure 4
Retinal vascular development in VEGF120/120 mice. (a) PECAM staining at P5. Dramatic impairment of vascular outgrowth. Arterioles and venules are morphologically indistinguishable. (b and c) EB2/CollIV at P5. Clusters of EB2-positive and -negative vessels alternate. (d and e) LacZ/Lec/SMA on VEGF120/120 × PECLacZ retina at P5. (d) Sparse blue cells near optic disc. (e) SMA immunoreactivity is undetectable. (f) EM at P5 shows capillary relaxation. (g) PECAM staining at P9 with more pronounced impairment in arteriolar than venular outgrowth. (h) Arterial SMA staining is fainter, while venules and capillaries have become strongly SMA positive at P14. (i) VEGF120/120 × Tie1LacZ retina at P9, with persisting dilated and tortuous hyaloid arteries. (j) Lec/GFAP. AC organization into a network is present in the vascularized area, but rapidly alters to the primitive bipolar shape and parallel organization in the more peripheral retina (arrow). (k) Unstained whole-mount preparation at P9 with retinal hemorrhages (arrows).
Figure 5
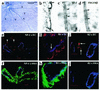
VEGF receptor expression in VEGF+/+ retinas. (a) Whole-mount in situ hybridization for NP-1 at P5. Expression is prominent in arterioles and discrete in venules. (b) In situ hybridization on retinal sections at P7 is suggestive of NP-1 expression in ECs as well as PECs (arrows). (c and d) High magnification of whole-mount in situ hybridization for (c) NP-1 and (d) PDGF receptor-β (PDGFRβ) illustrates that PECs are positive for PDGFRβ and suggest NP-1 expression in PECs (arrows). (e and j) Fluorescent triple immunostaining using lectin, anti-SMA, and anti-VEGFR on sections from VEGF+/+ eyes at P9. Lectin (blue), VEGFR (red), and SMA (green). (e and f) NP-1 is detectable in retinal neurons (arrowheads), but its expression is beyond the detection limit in vessels by immunostaining. (g and h) VEGFR-1 (R1) is expressed in vSMCs as well as ECs (arrow). (i and j) VEGFR-2 (R2) expression is only detectable in the ECs (arrows) in capillaries and venules.
Similar articles
- The role of vascular endothelial growth factor (VEGF) in vasculogenesis, angiogenesis, and hematopoiesis in zebrafish development.
Liang D, Chang JR, Chin AJ, Smith A, Kelly C, Weinberg ES, Ge R. Liang D, et al. Mech Dev. 2001 Oct;108(1-2):29-43. doi: 10.1016/s0925-4773(01)00468-3. Mech Dev. 2001. PMID: 11578859 - Role of the vascular endothelial growth factor isoforms in retinal angiogenesis and DiGeorge syndrome.
Stalmans I. Stalmans I. Verh K Acad Geneeskd Belg. 2005;67(4):229-76. Verh K Acad Geneeskd Belg. 2005. PMID: 16334858 Review. - Vascular endothelial growth factor: possible role in fetal development and placental function.
Cheung CY. Cheung CY. J Soc Gynecol Investig. 1997 Jul-Aug;4(4):169-77. J Soc Gynecol Investig. 1997. PMID: 9292845 Review. - Vascular endothelial growth factor (VEGF) and its receptors.
Neufeld G, Cohen T, Gengrinovitch S, Poltorak Z. Neufeld G, et al. FASEB J. 1999 Jan;13(1):9-22. FASEB J. 1999. PMID: 9872925 Review. - Regulation of retinal vascular endothelial growth factor and receptors in rabbits exposed to hyperoxia.
Ozaki NK, Beharry KD, Nishihara KC, Akmal Y, Ang JG, Sheikh R, Modanlou HD. Ozaki NK, et al. Invest Ophthalmol Vis Sci. 2002 May;43(5):1546-57. Invest Ophthalmol Vis Sci. 2002. PMID: 11980873
Cited by
- Capillary Network-Like Organization of Endothelial Cells in PEGDA Scaffolds Encoded with Angiogenic Signals via Triple Helical Hybridization.
Stahl PJ, Chan TR, Shen YI, Sun G, Gerecht S, Yu SM. Stahl PJ, et al. Adv Funct Mater. 2014 Jun 4;24(21):3213-3225. doi: 10.1002/adfm.201303217. Adv Funct Mater. 2014. PMID: 25541582 Free PMC article. No abstract available. - Anti-angiogenic Therapy for Retinal Disease.
Paulus YM, Sodhi A. Paulus YM, et al. Handb Exp Pharmacol. 2017;242:271-307. doi: 10.1007/164_2016_78. Handb Exp Pharmacol. 2017. PMID: 27783271 Free PMC article. - Anthrax lethal toxin suppresses high glucose induced VEGF over secretion through a post-translational mechanism.
Zhang WW, Wang X, Xie P, Yuan ST, Liu QH. Zhang WW, et al. Int J Ophthalmol. 2015 Jun 18;8(3):453-8. doi: 10.3980/j.issn.2222-3959.2015.03.04. eCollection 2015. Int J Ophthalmol. 2015. PMID: 26085990 Free PMC article. - Retinal Blood Vessel Distribution Correlates With the Peripapillary Retinal Nerve Fiber Layer Thickness Profile as Measured With GDx VCC and ECC.
Resch H, Pereira I, Weber S, Holzer S, Fischer G, Vass C. Resch H, et al. J Glaucoma. 2015 Jun-Jul;24(5):389-95. doi: 10.1097/IJG.0000000000000237. J Glaucoma. 2015. PMID: 25719231 Free PMC article. - VEGF-A stimulates lymphangiogenesis and hemangiogenesis in inflammatory neovascularization via macrophage recruitment.
Cursiefen C, Chen L, Borges LP, Jackson D, Cao J, Radziejewski C, D'Amore PA, Dana MR, Wiegand SJ, Streilein JW. Cursiefen C, et al. J Clin Invest. 2004 Apr;113(7):1040-50. doi: 10.1172/JCI20465. J Clin Invest. 2004. PMID: 15057311 Free PMC article.
References
- Carmeliet P, et al. Abnormal blood vessel development and lethality in embryos lacking a single VEGF allele. Nature. 1996;380:435–439. - PubMed
- Carmeliet P, Jain RK. Angiogenesis in cancer and other diseases. Nature. 2000;407:249–257. - PubMed
- Neufeld G, Cohen T, Gengrinovitch S, Poltorak Z. Vascular endothelial growth factor (VEGF) and its receptors. FASEB J. 1999;13:9–22. - PubMed
- Miao HQ, Klagsbrun M. Neuropilin is a mediator of angiogenesis. Cancer Metastasis Rev. 2000;19:29–37. - PubMed
- Soker S, Takashima S, Miao HQ, Neufeld G, Klagsbrun M. Neuropilin-1 is expressed by endothelial and tumor cells as an isoform-specific receptor for vascular endothelial growth factor. Cell. 1998;92:735–745. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases