Lesions of the nucleus basalis magnocellularis induced by 192 IgG-saporin block memory enhancement with posttraining norepinephrine in the basolateral amygdala - PubMed (original) (raw)
Lesions of the nucleus basalis magnocellularis induced by 192 IgG-saporin block memory enhancement with posttraining norepinephrine in the basolateral amygdala
Ann E Power et al. Proc Natl Acad Sci U S A. 2002.
Abstract
Extensive evidence indicates that drugs and stress hormones act in the basolateral amygdala (BLA) to modulate memory consolidation. The BLA projects to the nucleus basalis magnocellularis (NBM), which sends broad cholinergic projections to the neocortex. NBM-cortex projections have been implicated in learning, memory storage, and plasticity. The current study investigated whether the cholinergic NBM-cortex projections are involved in BLA-mediated modulation of memory consolidation. Bilateral cholinergic cell lesions of the NBM were induced in rats with infusions of 192 IgG-saporin (0.1 microg/0.5 microl per side). Additionally, cannulae were implanted bilaterally in the BLA. One week after surgery, the rats were trained in an inhibitory avoidance task and, immediately after training, norepinephrine (0.3 microg, 1.0 microg, or 3.0 microg in 0.2 microl) or vehicle (PBS) was infused bilaterally into the BLA. Norepinephrine infusions produced a dose-dependent enhancement of 48-h retention (0.3 microg and 1.0 microg doses enhanced) in nonlesioned rats but did not affect retention in NBM-lesioned rats. Choline acetyltransferase assays of frontal and occipital cortices confirmed the NBM lesions. These findings indicate that cholinergic NBM-cortex projections are required for BLA-mediated modulation of memory consolidation.
Figures
Figure 1
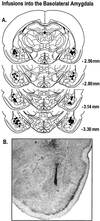
Only data from animals with posttraining infusion sites within the BLA were included in the analysis. (A) ♦ represents the infusion needle tip loci for a random sample of 30 rats included in the behavioral analysis. Coordinates indicate distance posterior to bregma. (B) Photomicrograph of a representative example of an infusion needle terminating within the BLA.
Figure 2
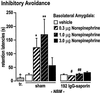
Intra-BLA infusions of norepinephrine immediately after IA training produce a dose-dependent enhancement of 48-h retention in sham-operated rats. The gray bar (tr.) indicates the mean training latency across all groups, which did not differ between any of the groups (P > 0.05). The mean training latency was significantly less than the retention latencies of the sham and lesion vehicle-infused groups ( , P < 0.05), which did not differ from each other (_P_ > 0.05). An ANOVA revealed significant main effects of the norepinephrine infusions, the 192 IgG-saporin lesion, and a significant norepinephrine infusion × lesion interaction effect (P < 0.05). The 0.3 μg (*, _P_ < 0.01) and the 1.0 μg (**, _P_ < 0.001) doses significantly enhanced retention relative to the vehicle-infused sham group. Rats with the 192 IgG-saporin NBM-lesions did not show memory enhancement with norepinephrine infusions. The mean retention latencies of the 0.3 μg (#, _P_ < 0.05) and the 1.0 μg (##, _P_ < 0.001) dose lesion groups were significantly shorter than those of their respective dose-matched sham control groups, but did not differ from that of the vehicle-infused sham or lesion groups (_P_ > 0.05). (The number of animals per group are as follows: sham-vehicle, 11; sham-norepinephrine 0.3 μg, 10; sham-norepinephrine 1.0 μg, 10; sham-norepinephrine 3.0 μg, 9; lesion-vehicle, 9; lesion-norepinephrine 0.3 μg, 7; lesion-norepinephrine 1.0 μg, 8; lesion-norepinephrine 3.0 μg, 14.)
, P < 0.05), which did not differ from each other (_P_ > 0.05). An ANOVA revealed significant main effects of the norepinephrine infusions, the 192 IgG-saporin lesion, and a significant norepinephrine infusion × lesion interaction effect (P < 0.05). The 0.3 μg (*, _P_ < 0.01) and the 1.0 μg (**, _P_ < 0.001) doses significantly enhanced retention relative to the vehicle-infused sham group. Rats with the 192 IgG-saporin NBM-lesions did not show memory enhancement with norepinephrine infusions. The mean retention latencies of the 0.3 μg (#, _P_ < 0.05) and the 1.0 μg (##, _P_ < 0.001) dose lesion groups were significantly shorter than those of their respective dose-matched sham control groups, but did not differ from that of the vehicle-infused sham or lesion groups (_P_ > 0.05). (The number of animals per group are as follows: sham-vehicle, 11; sham-norepinephrine 0.3 μg, 10; sham-norepinephrine 1.0 μg, 10; sham-norepinephrine 3.0 μg, 9; lesion-vehicle, 9; lesion-norepinephrine 0.3 μg, 7; lesion-norepinephrine 1.0 μg, 8; lesion-norepinephrine 3.0 μg, 14.)
Similar articles
- Behavioral, biochemical, histological, and electrophysiological effects of 192 IgG-saporin injections into the basal forebrain of rats.
Wenk GL, Stoehr JD, Quintana G, Mobley S, Wiley RG. Wenk GL, et al. J Neurosci. 1994 Oct;14(10):5986-95. doi: 10.1523/JNEUROSCI.14-10-05986.1994. J Neurosci. 1994. PMID: 7523630 Free PMC article. - 192 IgG-saporin: I. Specific lethality for cholinergic neurons in the basal forebrain of the rat.
Book AA, Wiley RG, Schweitzer JB. Book AA, et al. J Neuropathol Exp Neurol. 1994 Jan;53(1):95-102. doi: 10.1097/00005072-199401000-00012. J Neuropathol Exp Neurol. 1994. PMID: 8301325 - Comparison of site-specific injections into the basal forebrain on water maze and radial arm maze performance in the male rat after immunolesioning with 192 IgG saporin.
Dornan WA, McCampbell AR, Tinkler GP, Hickman LJ, Bannon AW, Decker MW, Gunther KL. Dornan WA, et al. Behav Brain Res. 1996 Dec;82(1):93-101. doi: 10.1016/s0166-4328(97)81112-6. Behav Brain Res. 1996. PMID: 9021074 - Immunolesion by 192IgG-saporin of rat basal forebrain cholinergic system: a useful tool to produce cortical cholinergic dysfunction.
Schliebs R, Rossner S, Bigl V. Schliebs R, et al. Prog Brain Res. 1996;109:253-64. doi: 10.1016/s0079-6123(08)62109-3. Prog Brain Res. 1996. PMID: 9009714 Review. - Memory consolidation and the amygdala: a systems perspective.
McGaugh JL. McGaugh JL. Trends Neurosci. 2002 Sep;25(9):456. doi: 10.1016/s0166-2236(02)02211-7. Trends Neurosci. 2002. PMID: 12183206 Review.
Cited by
- Dynamic behavior of the locus coeruleus during arousal-related memory processing in a multi-modal 7T fMRI paradigm.
Jacobs HI, Priovoulos N, Poser BA, Pagen LH, Ivanov D, Verhey FR, Uludağ K. Jacobs HI, et al. Elife. 2020 Jun 24;9:e52059. doi: 10.7554/eLife.52059. Elife. 2020. PMID: 32579109 Free PMC article. - Neuronal histamine and the memory of emotionally salient events.
Provensi G, Passani MB, Costa A, Izquierdo I, Blandina P. Provensi G, et al. Br J Pharmacol. 2020 Feb;177(3):557-569. doi: 10.1111/bph.14476. Epub 2018 Sep 18. Br J Pharmacol. 2020. PMID: 30110713 Free PMC article. Review. - Emotional enhancement of memory: how norepinephrine enables synaptic plasticity.
Tully K, Bolshakov VY. Tully K, et al. Mol Brain. 2010 May 13;3:15. doi: 10.1186/1756-6606-3-15. Mol Brain. 2010. PMID: 20465834 Free PMC article. Review. - Representational gain in cortical area underlies increase of memory strength.
Bieszczad KM, Weinberger NM. Bieszczad KM, et al. Proc Natl Acad Sci U S A. 2010 Feb 23;107(8):3793-8. doi: 10.1073/pnas.1000159107. Epub 2010 Feb 4. Proc Natl Acad Sci U S A. 2010. PMID: 20133679 Free PMC article. - Post-training intra-basolateral amygdala infusions of norepinephrine enhance consolidation of memory for contextual fear conditioning.
LaLumiere RT, Buen TV, McGaugh JL. LaLumiere RT, et al. J Neurosci. 2003 Jul 30;23(17):6754-8. doi: 10.1523/JNEUROSCI.23-17-06754.2003. J Neurosci. 2003. PMID: 12890768 Free PMC article.
References
- Gold P E, van Buskirk R. Behav Biol. 1978;23:509–520. - PubMed
- Sternberg D B, Issacs K, Gold P E, McGaugh J L. Behav Neural Biol. 1985;44:447–453. - PubMed
- Introini-Collison I B, McGaugh J L. Behav Neural Biol. 1986;45:358–365. - PubMed
- Brioni J D, Nagahara A H, McGaugh J L. Brain Res. 1989;487:105–112. - PubMed
- Brioni J D, McGaugh J L. Psychopharmacology. 1988;96:505–510. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- R01 AG017533/AG/NIA NIH HHS/United States
- R56 MH012526/MH/NIMH NIH HHS/United States
- AG17533/AG/NIA NIH HHS/United States
- MH12526/MH/NIMH NIH HHS/United States
- R01 MH012526/MH/NIMH NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources