Muscle-derived hematopoietic stem cells are hematopoietic in origin - PubMed (original) (raw)
Muscle-derived hematopoietic stem cells are hematopoietic in origin
Shannon L McKinney-Freeman et al. Proc Natl Acad Sci U S A. 2002.
Abstract
It has recently been shown that mononuclear cells from murine skeletal muscle contain the potential to repopulate all major peripheral blood lineages in lethally irradiated mice, but the origin of this activity is unknown. We have fractionated muscle cells on the basis of hematopoietic markers to show that the active population exclusively expresses the hematopoietic stem cell antigens Sca-1 and CD45. Muscle cells obtained from 6- to 8-week-old C57BL/6-CD45.1 mice and enriched for cells expressing Sca-1 and CD45 were able to generate hematopoietic but not myogenic colonies in vitro and repopulated multiple hematopoietic lineages of lethally irradiated C57BL/6-CD45.2 mice. These data show that muscle-derived hematopoietic stem cells are likely derived from the hematopoietic system and are a result not of transdifferentiation of myogenic stem cells but instead of the presence of substantial numbers of hematopoietic stem cells in the muscle. Although CD45-negative cells were highly myogenic in vitro and in vivo, CD45-positive muscle-derived cells displayed only very limited myogenic activity and only in vivo.
Figures
Figure 1
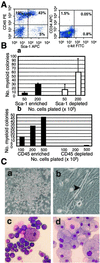
Sca-1-positive and -negative and CD45-positive muscle cells display in vitro hematopoietic progenitor activity_._ (A) Freshly isolated muscle-derived cells were stained with monoclonal antibodies recognizing CD45 and Sca-1 analyzed by flow cytometry. (B) Sca-1-positive and -negative muscle-derived cells were plated into methylcellulose and assessed for colony number 9 days after plating (a). CD45-positive and -negative cells were also plated into methylcellulose and assessed for colony number 9 days after plating (b). (C) Methylcellulose cultures of CD45-positive cells (a). Cultures of CD45-negative cells (b). The arrowhead indicates an actively twitching myofiber. Unfractionated muscle-derived colonies were picked, cytospun, and stained with Wright–Giemsa (c and d).
Figure 2
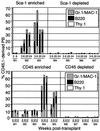
In vivo hematopoietic activity of Sca-1 and CD45-sorted populations of muscle-derived cells. Muscle was isolated from C57BL/6-CD45.1 mice and magnetically enriched for Sca-1 or CD45 expression. Positive and negative populations were transplanted into lethally irradiated C57BL/6-CD45.2 recipients along with 2 × 105 C57BL/6-CD45.2 WBM cells. PB of recipients was analyzed via FACS for CD45.1 and hematopoietic lineage markers at the indicated time points after transplantation. In the representative Sca-1 transplant shown, Thy.1 staining was not performed at 20 weeks after transplant and therefore does not appear.
Figure 3
In vivo myogenic potential of muscle-derived cells. Muscle-derived cells were purified from C57BL/6-ROSA26 (B–F) or C57/M_lacZ_ (H, I) mice by flow cytometry or magnetically followed by flow cytometry. Test populations were injected into the TA muscles of non-ROSA26 animals that had been injected with cardiotoxin 24 h earlier. The TA muscles were removed from recipients 2–3 weeks later, cryosectioned, and stained with X-Gal for β-galactosidase expression. (Bar = 50 μM.) (A) Injured muscle injected with HBSS. (B) Muscle injected with unfractionated muscle-derived cells. The large blue tracts are fibers regenerated from Rosa26-derived cells. (C) Incorporation of Sca-1-positive muscle cells. (D) Incorporation of Sca-1-negative muscle cells. (E) Incorporation of Sca-1+/CD45+ cells. (F) Incorporation of Sca-1+/CD45− cells. (G) Section of unmanipulated C57/M_lacZ_ muscle. Note the nuclear localization of lacZ at the edges of the fibers shown in cross section. (H) CD45-positive C57/M_lacZ_-derived cells injected into regenerating muscle and sectioned longitudinally. (I) CD45-negative C57/M_lacZ_ incorporation.
Similar articles
- Hematopoietic potential of murine skeletal muscle-derived CD45(-)Sca-1(+)c-kit(-) cells.
Howell JC, Yoder MC, Srour EF. Howell JC, et al. Exp Hematol. 2002 Aug;30(8):915-24. doi: 10.1016/s0301-472x(02)00872-x. Exp Hematol. 2002. PMID: 12160843 - Myogenic specification of side population cells in skeletal muscle.
Asakura A, Seale P, Girgis-Gabardo A, Rudnicki MA. Asakura A, et al. J Cell Biol. 2002 Oct 14;159(1):123-34. doi: 10.1083/jcb.200202092. Epub 2002 Oct 14. J Cell Biol. 2002. PMID: 12379804 Free PMC article. - Hematopoietic potential of stem cells isolated from murine skeletal muscle.
Jackson KA, Mi T, Goodell MA. Jackson KA, et al. Proc Natl Acad Sci U S A. 1999 Dec 7;96(25):14482-6. doi: 10.1073/pnas.96.25.14482. Proc Natl Acad Sci U S A. 1999. PMID: 10588731 Free PMC article. - Altered phenotype and reduced function of muscle-derived hematopoietic stem cells.
McKinney-Freeman SL, Majka SM, Jackson KA, Norwood K, Hirschi KK, Goodell MA. McKinney-Freeman SL, et al. Exp Hematol. 2003 Sep;31(9):806-14. doi: 10.1016/s0301-472x(03)00186-3. Exp Hematol. 2003. PMID: 12962727 - Stem cell plasticity in muscle and bone marrow.
Goodell MA, Jackson KA, Majka SM, Mi T, Wang H, Pocius J, Hartley CJ, Majesky MW, Entman ML, Michael LH, Hirschi KK. Goodell MA, et al. Ann N Y Acad Sci. 2001 Jun;938:208-18; discussion 218-20. doi: 10.1111/j.1749-6632.2001.tb03591.x. Ann N Y Acad Sci. 2001. PMID: 11458510 Review.
Cited by
- The existence of myocardial repair: mechanistic insights and enhancements.
Schoenfeld M, Frishman WH, Leri A, Kajstura J, Anversa P. Schoenfeld M, et al. Cardiol Rev. 2013 May-Jun;21(3):111-20. doi: 10.1097/CRD.0b013e318289d7a9. Cardiol Rev. 2013. PMID: 23568056 Free PMC article. Review. - Stem cell plasticity: from transdifferentiation to macrophage fusion.
Camargo FD, Chambers SM, Goodell MA. Camargo FD, et al. Cell Prolif. 2004 Feb;37(1):55-65. doi: 10.1111/j.1365-2184.2004.00300.x. Cell Prolif. 2004. PMID: 14871237 Free PMC article. Review. - CXCR4 enhances engraftment of muscle progenitor cells.
Perez AL, Bachrach E, Illigens BM, Jun SJ, Bagden E, Steffen L, Flint A, McGowan FX, Del Nido P, Montecino-Rodriguez E, Tidball JG, Kunkel LM. Perez AL, et al. Muscle Nerve. 2009 Oct;40(4):562-72. doi: 10.1002/mus.21317. Muscle Nerve. 2009. PMID: 19760789 Free PMC article. - BMP9-induced osteogenetic differentiation and bone formation of muscle-derived stem cells.
Xiang L, Liang C, Zhen-Yong K, Liang-Jun Y, Zhong-Liang D. Xiang L, et al. J Biomed Biotechnol. 2012;2012:610952. doi: 10.1155/2012/610952. Epub 2012 Jan 26. J Biomed Biotechnol. 2012. PMID: 22319244 Free PMC article. - Regulation of myogenic progenitor proliferation in human fetal skeletal muscle by BMP4 and its antagonist Gremlin.
Frank NY, Kho AT, Schatton T, Murphy GF, Molloy MJ, Zhan Q, Ramoni MF, Frank MH, Kohane IS, Gussoni E. Frank NY, et al. J Cell Biol. 2006 Oct 9;175(1):99-110. doi: 10.1083/jcb.200511036. Epub 2006 Oct 2. J Cell Biol. 2006. PMID: 17015616 Free PMC article.
References
- Gussoni E, Soneoka Y, Strickland C D, Buzney E A, Khan M K, Flint A F, Kunkel L M, Mulligan R C. Nature (London) 1999;401:390–394. - PubMed
- Lagasse E, Connors H, Al-Dhalimy M, Reitsma M, Dohse M, Osborne L, Wang X, Finegold M, Weissman I L, Grompe M. Nat Med. 2000;6:1229–1234. - PubMed
- Krause D S, Theise N D, Collector M I, Henegariu O, Hwang S, Gardner R, Neutzel S, Sharkis S J. Cell. 2001;105:369–377. - PubMed
- Bjornson C R R, Rietze R L, Reynolds B A, Magli M C, Vescovi A L. Science. 1999;283:534–537. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- DK58192/DK/NIDDK NIH HHS/United States
- T32 AI007495/AI/NIAID NIH HHS/United States
- TGT00D01/TI_/Telethon/Italy
- R01 DK058192/DK/NIDDK NIH HHS/United States
- TGT06S01/TI_/Telethon/Italy
- T32 AI07495/AI/NIAID NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous