The Brucella suis virB operon is induced intracellularly in macrophages - PubMed (original) (raw)
The Brucella suis virB operon is induced intracellularly in macrophages
Maria Laura Boschiroli et al. Proc Natl Acad Sci U S A. 2002.
Abstract
A type IV secretion system similar to the VirB system of the phytopathogen Agrobacterium tumefaciens is essential for the intracellular survival and multiplication of the mammalian pathogen Brucella. Reverse transcriptase-PCR showed that the 12 genes encoding the Brucella suis VirB system form an operon. Semiquantitative measurements of virB mRNA levels by slot blotting showed that transcription of the virB operon, but not the flanking genes, is regulated by environmental factors in vitro. Flow cytometry used to measure green fluorescent protein expression from the virB promoter confirmed the data from slot blots. Fluorescence-activated cell sorter analysis and fluorescence microscopy showed that the virB promoter is induced in macrophages within 3 h after infection. Induction only occurred once the bacteria were inside the cells, and phagosome acidification was shown to be the major signal inducing intracellular expression. Because phagosome acidification is essential for the intracellular multiplication of Brucella, we suggest that it is the signal that triggers the secretion of unknown effector molecules. These effector molecules play a role in the remodeling of the phagosome to create the unique intracellular compartment in which Brucella replicates.
Figures
Figure 1
Transcriptional organization of the B. suis virB region. (A) Organization of the virB region showing the regions amplified by the primer pairs. (B) Agarose gel of the RT-PCR amplification products. For each primer pair, three lanes are shown [a, positive control using genomic DNA templates; b, RT-PCR; c, negative control (RNA, no RT)] to asses DNA contamination in RNA preparations. The molecular weight marker is the 1-kb ladder (Life Technologies). (C) Hybridization of different virB probes to RNA from B. suis 1330 [wild type (WT)] and 1330 _virB1_∷Kan showing a polar effect on downstream genes.
Figure 2
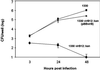
VirB12 is essential for Brucella virulence. Differentiated THP1 macrophage-like cells were infected with B. suis 1330 (wild type), 1330 _virB12_∷ Kan, and 1330 virB12_∷Kan (pBB_virB) as described in Experimental Procedures. Data points are the geometric mean of three wells with standard error. This data set is representative of three independent experiments with similar results. CFU, colony-forming unit.
Figure 3
Measurement of virB expression. (A) Measurement of gfp expression from the virB promoter. A1, bacteria grown to stationary phase in TSB; A2, bacteria recovered from J774 macrophages 3 h postinfection; A3, bacteria incubated for 3 h in E medium, pH 4.5; A4, bacteria recovered after 3 h contact with cytochalasin D-treated (10 μg/ml) J774 macrophages; A5, bacteria recovered from bafilomycin A-treated (100 nM) macrophages 3 h postinfection; A6, bacteria recovered from ammonium chloride-treated (30 mM) macrophages 3 h postinfection. In all panels, expression from pvirB is shown in blue, expression from the empty vector is shown in red, and expression from a constitutive promoter is shown in green. Where appropriate, background fluorescence of the macrophages is shown in yellow. (B) Examination of gfp expression from pvirB by fluorescence microscopy. J774 macrophages infected for 3 h with bacteria expressing GFP constitutively (B1 and B3) or from pvirB (B2 and B4) in the presence (B3 and B4) or absence (B1 and B2) of bafilomycin (100 nM). (C) Environmental regulation of virB transcription. Slot blots of total RNA extracted from B. suis grown in different conditions were hybridized with a virB5 probe as described in Experimental Procedures. For simplicity, a single RNA dilution is shown for each condition. (Top) Medium: bacteria were grown to early exponential phase in rich (TSB) or minimal medium pH 7.0 with glucose (E). (Middle) Growth phase: bacteria were grown in minimal medium at pH 7.0 with glucose and harvested at an optical density at 600 nm of 0.015 (lag), 0.1 (early exponential), 0.25 (late exponential), 0.45 (early stationary), and 0.55 (late stationary). (Bottom) pH and temperature: bacteria were grown to early exponential phase in minimal medium with glucose at the indicated pH and temperature.
Similar articles
- Integration host factor is involved in transcriptional regulation of the Brucella abortus virB operon.
Sieira R, Comerci DJ, Pietrasanta LI, Ugalde RA. Sieira R, et al. Mol Microbiol. 2004 Nov;54(3):808-22. doi: 10.1111/j.1365-2958.2004.04316.x. Mol Microbiol. 2004. PMID: 15491369 - Identification of a new virulence factor, BvfA, in Brucella suis.
Lavigne JP, Patey G, Sangari FJ, Bourg G, Ramuz M, O'Callaghan D, Michaux-Charachon S. Lavigne JP, et al. Infect Immun. 2005 Sep;73(9):5524-9. doi: 10.1128/IAI.73.9.5524-5529.2005. Infect Immun. 2005. PMID: 16113268 Free PMC article. - The stringent response mediator Rsh is required for Brucella melitensis and Brucella suis virulence, and for expression of the type IV secretion system virB.
Dozot M, Boigegrain RA, Delrue RM, Hallez R, Ouahrani-Bettache S, Danese I, Letesson JJ, De Bolle X, Köhler S. Dozot M, et al. Cell Microbiol. 2006 Nov;8(11):1791-802. doi: 10.1111/j.1462-5822.2006.00749.x. Epub 2006 Jun 27. Cell Microbiol. 2006. PMID: 16803581 - Type IV secretion and Brucella virulence.
Boschiroli ML, Ouahrani-Bettache S, Foulongne V, Michaux-Charachon S, Bourg G, Allardet-Servent A, Cazevieille C, Lavigne JP, Liautard JP, Ramuz M, O'Callaghan D. Boschiroli ML, et al. Vet Microbiol. 2002 Dec 20;90(1-4):341-8. doi: 10.1016/s0378-1135(02)00219-5. Vet Microbiol. 2002. PMID: 12414154 Review.
Cited by
- The putative type 4 secretion system effector BspD is involved in maintaining envelope integrity of the pathogen Brucella.
Ketterer M, Chiquet P, Esposito M, Sedzicki J, Québatte M, Dehio C. Ketterer M, et al. mSphere. 2024 Nov 21;9(11):e0023224. doi: 10.1128/msphere.00232-24. Epub 2024 Oct 10. mSphere. 2024. PMID: 39387552 Free PMC article. - Copper sensing transcription factor ArsR2 regulates VjbR to sustain virulence in Brucella abortus.
Zhi F, Liu K, Geng H, Su M, Xu J, Fu L, Ma K, Gao P, Yuan L, Chu Y. Zhi F, et al. Emerg Microbes Infect. 2024 Dec;13(1):2406274. doi: 10.1080/22221751.2024.2406274. Epub 2024 Sep 25. Emerg Microbes Infect. 2024. PMID: 39295505 Free PMC article. - Evasion of host defense by Brucella.
Yang J, Wang Y, Hou Y, Sun M, Xia T, Wu X. Yang J, et al. Cell Insight. 2023 Dec 17;3(1):100143. doi: 10.1016/j.cellin.2023.100143. eCollection 2024 Feb. Cell Insight. 2023. PMID: 38250017 Free PMC article. Review. - The Brucella Effector Protein BspF Regulates Apoptosis through the Crotonylation of p53.
Lin R, Li A, Li Y, Shen R, Du F, Zheng M, Zhu J, Chen J, Jiang P, Zhang H, Liu J, Chen X, Chen Z. Lin R, et al. Microorganisms. 2023 Sep 15;11(9):2322. doi: 10.3390/microorganisms11092322. Microorganisms. 2023. PMID: 37764165 Free PMC article. - Unraveling the Role of the Zinc-Dependent Metalloproteinase/HTH-Xre Toxin/Antitoxin (TA) System of Brucella abortus in the Oxidative Stress Response: Insights into the Stress Response and Virulence.
Gómez LA, Molina RE, Soto RI, Flores MR, Coloma-Rivero RF, Montero DA, Oñate ÁA. Gómez LA, et al. Toxins (Basel). 2023 Aug 31;15(9):536. doi: 10.3390/toxins15090536. Toxins (Basel). 2023. PMID: 37755962 Free PMC article.
References
- Covacci A, Rappuoli R. Mol Microbiol. 1993;8:429–434. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources