Role of an intact splenic microarchitecture in early lymphocytic choriomeningitis virus production - PubMed (original) (raw)
Role of an intact splenic microarchitecture in early lymphocytic choriomeningitis virus production
Stefan Müller et al. J Virol. 2002 Mar.
Abstract
An acute infection with lymphocytic choriomeningitis virus (LCMV) is efficiently controlled by the cytotoxic-T-cell (CTL) response of the host, and LCMV titers in the spleen and peripheral solid organs usually fall sharply after day 4 to 6 postinfection. Surprisingly, infection of immunodeficient recombination-activating gene 2-deficient (RAG2-/-) mice with 5 x 10(2) PFU of LCMV-WE causes about 80-fold-lower LCMV titers in the spleen on day 4 postinfection compared with C57BL/6 control mice. This could not be attributed to NK cell activity, since common gamma-chain-deficient RAG2-/- mice lacking NK cells show low LCMV titers comparable to those for RAG2-/- mice. Furthermore, the reduced early LCMV production in spleens could not be explained by an enhanced gamma interferon production in RAG2-/- mice. Analysis of mutant mice exhibiting various defects in the splenic microarchitecture, including (i) tumor necrosis factor alpha-negative (TNF-alpha-/-), lymphotoxin alpha-negative (LTalpha-/-), B-cell-deficient muMT mice, (ii) immunoglobulin M-negative mice, and (iii) RAG2-/- mice reconstituted with wild-type versus TNF-alpha-/- LTalpha-/- B cells, revealed a clear correlation between an intact splenic marginal zone, rapid early replication of LCMV in the spleen, and efficient CTL induction. These results suggest that by the preferential infection of the highly organized splenic microarchitecture, LCMV seems to successfully exploit one of the key elements in the chain of the adaptive immune system. Not only does the early tropism of LCMV for the splenic marginal zone trigger a potent immune response, but at the same time the marginal zone may also become a target of early CTL-mediated immunopathology that impairs immune responsiveness.
Figures
FIG. 1.
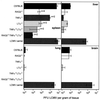
Early LCMV production is reduced in the spleens of RAG2−/− mice. (A) Kinetics of LCMV-WE titers in the spleens of C57BL/6 mice and RAG2−/− mice following i.v. infection with 500 PFU of LCMV-WE. d, days. (B) Splenic LCMV titers on day 4 after LCMV-WE infection (500 PFU, i.v.) in C57BL/6, RAG2−/−, and RAG2−/− γc−/− mice (mean values for three to five mice per time point ± standard errors of the means; asterisks indicate levels below the detection limit).
FIG. 2.
LCMV titers in the spleen, liver, lung, and brain in mutant and wild-type mice. C57BL/6, RAG2−/−, TNF-α−/−, LTα−/−, TNF-α−/− LTα−/−, and RAG2−/− TNF-α−/− LTα−/− were infected i.v. with a low dose (500 PFU) of LCMV-WE. Four days later LCMV in the indicated organs was titrated. For comparison, LCMV titers in organs from neonatally LCMV-infected C57BL/6 mice (LCMV carrier mice) were also assessed. Bars represent mean values from n animals. Error bars show the range (for n = 2) or standard error of the mean (for n > 2).
FIG. 3.
Decreased LCMV GP-specific in situ hybridization signals in spleens of mutant mice. C57BL/6, RAG2−/−, TNF-α−/−, LTα−/−, and TNF-α−/− LTα−/− mice were infected with 500 PFU of LCMV-WE i.v., and spleens were harvested 4 days later. Cryosections of spleens were used for hybridization with an RNA probe specific for LCMV GP mRNA. Representative frozen tissue sections of a noninfected C57BL/6 mouse (a) and day 4 infected C57BL/6 (b), RAG2−/− (c), TNF-α−/− (d), LTα−/− (e), and TNF-α−/− LTα−/− (f) mice, hybridized with an LCMV GP-specific RNA probe (mRNA-specific cells, black silver grains), are shown (magnification, ×125).
FIG. 4.
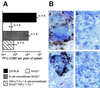
Presence of MAdCAM-1+ reticular endothelial cells in the splenic marginal zone and of ERTR-9+ marginal zone macrophages but lack (or much less) of N418-positive dendritic cells is associated with extensive early virus production in the spleen following LCMV-WE infection (500 PFU, i.v.). Representative examples of spleen sections from C57BL/6 mice at 24 h postinfection (5 × 103 PFU of LCMV per g of spleen) (A and B) and 4 days postinfection (8.2 × 106 PFU of LCMV per g of spleen) (C and D), from a RAG2−/− mouse (7 × 104 PFU of LCMV per g of spleen) (E) and an LTα−/− mouse (8 × 104 PFU of LCMV per g of spleen) (F) at 4 days postinfection, and from a RAG2−/− mouse (1.6 × 103 PFU of LCMV per g of spleen) (G) and a C57BL/6 mouse (1.5 × 105 PFU of LCMV per g of spleen) (H) at 48 h postinfection, with immunostaining for MAdCAM-1 (A, C, E, and F), ERTR-9 (B and D), or CD11c (N418) (G and H) (positive reaction in red), were hybridized in situ with 35S-labeled RNA probes specific for LCMV GP (mRNA-positive cells, black silver grains). Magnifications, ×125 (A to D) and ×250 (E to H).
FIG. 5.
Reconstitution of the splenic follicular microarchitecture with wild-type B cells restores impaired, early LCMV production in RAG2−/− mice. (A) Various mice or chimeras were infected with 500 PFU of LCMV-WE i.v., and 4 days later LCMV titers were determined. Bars represent mean values for n animals. Error bars show the range (for n = 2) or standard error of the mean (for n = 4). (B) Representative examples of immunohistochemical analysis with MAdCAM-1+ antibodies and LCMV GP-specific in situ hybridization of C57BL/6 (a), RAG2−/− (b), B-cell-reconstituted RAG2−/− (c), and TNF-α−/− LTα−/− B-cell-reconstituted RAG2−/− TNF-α−/− LTα−/− (d) mice at 4 days after LCMV-WE infection. Magnification, ×125.
FIG. 6.
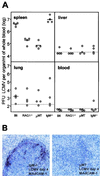
Early splenic LCMV production after low-dose infection i.v. with 5 × 102 PFU of LCMV-WE depends on an intact splenic architecture but not on IgM in serum. (A) Four mice of each indicated strain were infected with 500 PFU of LCMV i.v., and the LCMV-WE titers in the indicated organs from individual mice are shown. LCMV detection limits for each organ are indicated as dotted lines. (B) Spleen sections from an IgM−/− C57BL/6 mouse (with no serum IgM but all other Ig classes) (left) and a μMT mouse (no serum Ig) at 4 days postinfection, immunostained for MAdCAM-1 (positive reaction in red) and hybridized in situ with 35S-labeled RNA probes specific for LCMV GP. Magnification, ×250.
Similar articles
- Type 1 IFN deficiency in the absence of normal splenic architecture during lymphocytic choriomeningitis virus infection.
Louten J, van Rooijen N, Biron CA. Louten J, et al. J Immunol. 2006 Sep 1;177(5):3266-72. doi: 10.4049/jimmunol.177.5.3266. J Immunol. 2006. PMID: 16920967 - Destruction of lymphoid organ architecture and hepatitis caused by CD4+ T cells.
Matter MS, Hilmenyuk T, Claus C, Marone R, Schürch C, Tinguely M, Terracciano L, Luther SA, Ochsenbein AF. Matter MS, et al. PLoS One. 2011;6(9):e24772. doi: 10.1371/journal.pone.0024772. Epub 2011 Sep 23. PLoS One. 2011. PMID: 21966366 Free PMC article. - Low-affinity cytotoxic T-lymphocytes require IFN-gamma to clear an acute viral infection.
Von Herrath MG, Coon B, Oldstone MB. Von Herrath MG, et al. Virology. 1997 Mar 17;229(2):349-59. doi: 10.1006/viro.1997.8442. Virology. 1997. PMID: 9126248 - Lymphocytic choriomeningitis virus injures the developing brain: effects and mechanisms.
Bonthius DJ. Bonthius DJ. Pediatr Res. 2024 Jan;95(2):551-557. doi: 10.1038/s41390-023-02985-5. Epub 2024 Jan 5. Pediatr Res. 2024. PMID: 38182822 Review. - Lymphocytic choriomeningitis infection of the central nervous system.
Kang SS, McGavern DB. Kang SS, et al. Front Biosci. 2008 May 1;13:4529-43. doi: 10.2741/3021. Front Biosci. 2008. PMID: 18508527 Free PMC article. Review.
Cited by
- Inhibition of the type I interferon antiviral response during arenavirus infection.
Borrow P, Martínez-Sobrido L, de la Torre JC. Borrow P, et al. Viruses. 2010 Nov;2(11):2443-80. doi: 10.3390/v2112443. Epub 2010 Nov 5. Viruses. 2010. PMID: 21994626 Free PMC article. - Host-Viral Infection Maps Reveal Signatures of Severe COVID-19 Patients.
Bost P, Giladi A, Liu Y, Bendjelal Y, Xu G, David E, Blecher-Gonen R, Cohen M, Medaglia C, Li H, Deczkowska A, Zhang S, Schwikowski B, Zhang Z, Amit I. Bost P, et al. Cell. 2020 Jun 25;181(7):1475-1488.e12. doi: 10.1016/j.cell.2020.05.006. Epub 2020 May 8. Cell. 2020. PMID: 32479746 Free PMC article. - Type I interferon suppresses virus-specific B cell responses by modulating CD8+ T cell differentiation.
Moseman EA, Wu T, de la Torre JC, Schwartzberg PL, McGavern DB. Moseman EA, et al. Sci Immunol. 2016 Oct;1(4):eaah3565. doi: 10.1126/sciimmunol.aah3565. Epub 2016 Oct 21. Sci Immunol. 2016. PMID: 27812556 Free PMC article. - Splenic fibroblastic reticular cells orchestrate dendritic cell maturation and facilitate CD8+ T cell priming and protective memory.
Alexandre YO, Potemkin N, Schienstock D, Duchamp B, Poch A, Christo SN, Li S, Qin L, Beattie L, Utzschneider DT, Ono M, Schröder J, Mackay LK, Mueller SN. Alexandre YO, et al. Sci Adv. 2025 Jun 6;11(23):eadt5939. doi: 10.1126/sciadv.adt5939. Epub 2025 Jun 4. Sci Adv. 2025. PMID: 40465738 Free PMC article. - Fatal Lassa fever in cynomolgus monkeys is associated with systemic viral dissemination and inflammation.
Hortion J, Perthame E, Lafoux B, Soyer L, Reynard S, Journeaux A, Germain C, Lopez-Maestre H, Pietrosemoli N, Baillet N, Croze S, Rey C, Legras-Lachuer C, Baize S. Hortion J, et al. PLoS Pathog. 2024 Dec 9;20(12):e1012768. doi: 10.1371/journal.ppat.1012768. eCollection 2024 Dec. PLoS Pathog. 2024. PMID: 39652618 Free PMC article.
References
- Althage, A., B. Odermatt, D. Moskophidis, T. Kundig, U. Hoffman-Rohrer, H. Hengartner, and R. M. Zinkernagel. 1992. Immunosuppression by lymphocytic choriomeningitis virus infection: competent effector T and B cells but impaired antigen presentation. Eur. J. Immunol. 22:1803-1812. - PubMed
- Battegay, M., S. Cooper, A. Althage, J. Banziger, H. Hengartner, and R. M. Zinkernagel. 1991. Quantification of lymphocytic choriomeningitis virus with an immunological focus assay in 24- or 96-well plates. J. Virol. Methods 33:191-198. (Erratum, 35:115, 1991; erratum, 38:263, 1992.) - PubMed
- Berger, D. P., D. Naniche, M. T. Crowley, P. A. Koni, R. A. Flavell, and M. B. Oldstone. 1999. Lymphotoxin-beta-deficient mice show defective antiviral immunity. Virology 260:136-147. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources