Tpr is localized within the nuclear basket of the pore complex and has a role in nuclear protein export - PubMed (original) (raw)
Tpr is localized within the nuclear basket of the pore complex and has a role in nuclear protein export
Phyllis Frosst et al. J Cell Biol. 2002.
Abstract
Tpr is a coiled-coil protein found near the nucleoplasmic side of the pore complex. Since neither the precise localization of Tpr nor its functions are well defined, we generated antibodies to three regions of Tpr to clarify these issues. Using light and EM immunolocalization, we determined that mammalian Tpr is concentrated within the nuclear basket of the pore complex in a distribution similar to Nup153 and Nup98. Antibody localization together with imaging of GFP-Tpr in living cells revealed that Tpr is in discrete foci inside the nucleus similar to several other nucleoporins but is not present in intranuclear filamentous networks (Zimowska et al., 1997) or in long filaments extending from the pore complex (Cordes et al., 1997) as proposed. Injection of anti-Tpr antibodies into mitotic cells resulted in depletion of Tpr from the nuclear envelope without loss of other pore complex basket proteins. Whereas nuclear import mediated by a basic amino acid signal was unaffected, nuclear export mediated by a leucine-rich signal was retarded significantly. Nuclear injection of anti-Tpr antibodies in interphase cells similarly yielded inhibition of protein export but not import. These results indicate that Tpr is a nucleoporin of the nuclear basket with a role in nuclear protein export.
Figures
Figure 1.
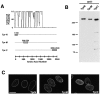
Characterization of antibodies to three Tpr fragments. (A) Antibodies against Tpr were made to NH2- and COOH-terminal fragments and to an internal fragment spanning a break in the predicted coiled-coil region as indicated. (B) HeLa cell extracts were probed by Western blotting with anti-TprN, anti-TprM, and anti-TprC antibodies. (C) HeLa cells were stained with the three anti-Tpr antibodies for immunofluorescence visualization and examined by confocal microscopy. Bar, 5 μm.
Figure 2.
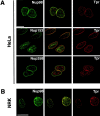
Localization of Tpr in cultured mammalian cells. Immunofluorescence staining followed by deconvolution microscopy was used to visualize Tpr (red) in single optical sections HeLa and NRK cells as indicated. Cells are colabeled with antibodies to either Nup98, Nup153, or Nup358 (green). The merged images are shown in the middle. Bars, 5 μm.
Figure 3.
Localization of Tpr and Nup98 in HeLa cells by immunofluorescence staining and deconvolution microscopy. (A) Volume projection of 20 sequential 0.2 μm z-sections stained with anti-Tpr antibodies (red). Panels are shown at 0 and 90° to the plane of the slide. (B) Serial z-sections through a nucleus at 0.4-μm intervals. Selected individual foci that successively increase and decrease in intensity are marked by arrows; numbers are in μm. Bars, 5 μm.
Figure 4.
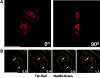
Imaging of GFP-Tpr in HeLa cells. Cells were examined 50 h after transfection using confocal (A) or deconvolution (B and C) techniques. (A) Cells were fixed and immunostained with anti-TprC (GFP fluorescence, left, green; anti-TprC, right, red). (B and C) Live cell imaging of GFP-Tpr. Different fields of cells are shown on the left and right. Single optical sections though the middle of the nucleus are shown in A and B; a projected stack of sections encompassing the entire depth of the nuclei is shown in C. See also videos 1 and 2 available at
http://www.jcb.org/cgi/content/full/jcb.200106046/DC1
. Bars, 5 μm.
Figure 5.
Immuno-EM localization of Tpr in nuclei of cultured cells and liver. NPCs are indicated by arrows; gold is seen at the nuclear face of NPCs and also either as single particles (arrowheads) or as clusters in the nuclear interior (asterisks). (A) Tpr was localized by preembedding labeling of rat liver nuclei permeabilized by freeze thawing. (B and C) Tpr was localized by preembedding labeling of HeLa cells, which were permeabilized by freeze thawing. 5-nm gold particles label TprM, and 10-nm gold particles label TprN. (D and E) Tpr was detected by immunogold labeling of ultrathin cryosections of rat liver. Darkly staining matter at the nuclear periphery is condensed chromatin. Bars, 200 nm.
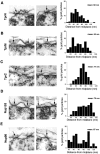
Figure 6.
Immunoelectron microscopic localization of Tpr at the NPC in HeLa cells. Indirect immunogold labeling was performed for Tpr and the nuclear basket proteins Nup153 and Nup98 in HeLa cells using the preembedding technique. (Left) Gallery of NPCs (arrows) decorated with various antibodies as indicated. (Right) Quantification of gold particle distance from the NPC midplane. (A) anti-TprN, n = 124 particles; (B) anti-TprM, n = 258 particles; (C) anti-TprC, n = 105 particles; (D) anti-TprN and Nup153 n = 166 particles for 153; and (E) anti-Nup98, n = 100 particles. The average distance from the midplane of the NPC is indicated as mean. Bars, 50 nm.
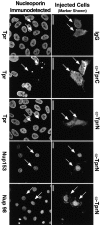
Figure 7.
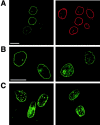
Immunofluorescent localization of nucleoporins 4 h after microinjection of anti-TprN, anti-TprC, or control rabbit IgG into NRK cells at mitosis. The injection marker (Alexa fluor 488–conjugated BSA) and rhodamine-coupled secondary antibodies specific to the species in which the injected antibody was raised, which recognize the injected antibodies, identify injected cells (arrows), and are imaged on the right. The labels on the right designate the injected antibody, and the labels on the left indicate which nucleoporin was stained for, using antibodies raised in a different host species and directed against a different segment of Tpr than the injected antibody. The immunodetected nucleoporins are shown on the left. (Guinea pig anti-TprN antibodies and rabbit anti-Nup98 or Nup153 were used for detection, and the complementary species of antibody was used for injection.) Images were acquired by confocal microscopy. Bars, 5 μm.
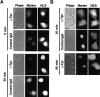
Figure 8.
Analysis of nuclear import and export in NRK cells injected with control IgG or depleted of Tpr by mitotic microinjection of anti-TprN. (A) Nuclear import of GST-NLS cargo (as described in Materials and methods) 5 and 30 min after cargo injection. The localization of the injection marker (Alexa fluor 488–conjugated BSA) and cargo (NLS) are indicated. (B) Nuclear export of GST-NES at 30 min (for IgG and Tpr) and 60 min (for Tpr only) after cargo injection. Images representative of the three classifications for transport signal are shown: NLS, shows N > C (A, top); NES, shows C > N (B, middle); and NES, shows N = C (B, bottom). Texas red–conjugated BSA was the mitotic cell injection marker used in the 30-min time point for the anti-Tpr injections, and Alexa fluor 488–conjugated BSA was the injection marker used in the 30-min time point for the control IgG injection and the 60-min time point for the anti-Tpr injection. After mitosis, Texas red–conjugated BSA was observed to distribute between the cytoplasm and the nucleus, whereas Alexa fluor–conjugated BSA was observed to segregate to the nucleus. Bars, 5 μm.
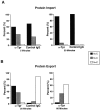
Figure 9.
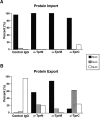
Analysis of nuclear import and export in interphase NRK cells microinjected with control antibodies or depleted of Tpr by mitotic microinjection of anti-TprN antibodies. Cells subsequently injected with a fluorescently labeled cargo were fixed at the times indicated and examined by immunofluorescence microscopy. Cells were classified as having nuclear fluorescence greater than (N > C), equal to (N = C), or less than (N < C) cytoplasmic fluorescence. (A) Transport of a basic-type NLS import cargo (GST-NLS) examined at 5 and 30 min after cargo injection. (B) Transport of a leucine-rich NES cargo (GST-NES) at 30 and 60 min after cargo injection in cells injected at mitosis with anti-Tpr antibodies and at 30 min after cargo injection in cells injected at mitosis with control IgG. Between 30 and 50 cells were analyzed for each time point.
Figure 10.
Analysis of nuclear import and export in interphase cells injected with anti-Tpr or with control IgG antibodies. Cells were fixed, imaged, and scored for transport as in the legend to Fig. 9. (A) Anti-TprN, anti-TprM, anti-TprC, or control IgG was injected into the nuclei of NRK cells, and after 20–30 min cells were cytoplasmically injected with a basic-type NLS import cargo (GST-NLS). (B) Anti-TprN, anti-TprM, anti-TprC, or control IgG were coinjected into the nuclei of NRK cells with a leucine-rich NES cargo (GST-NES). Between 30 and 50 cells were analyzed for each time point.
Similar articles
- Nucleoporins as components of the nuclear pore complex core structure and Tpr as the architectural element of the nuclear basket.
Krull S, Thyberg J, Björkroth B, Rackwitz HR, Cordes VC. Krull S, et al. Mol Biol Cell. 2004 Sep;15(9):4261-77. doi: 10.1091/mbc.e04-03-0165. Epub 2004 Jun 30. Mol Biol Cell. 2004. PMID: 15229283 Free PMC article. - Localization of nucleoporin Tpr to the nuclear pore complex is essential for Tpr mediated regulation of the export of unspliced RNA.
Rajanala K, Nandicoori VK. Rajanala K, et al. PLoS One. 2012;7(1):e29921. doi: 10.1371/journal.pone.0029921. Epub 2012 Jan 13. PLoS One. 2012. PMID: 22253824 Free PMC article. - Novel vertebrate nucleoporins Nup133 and Nup160 play a role in mRNA export.
Vasu S, Shah S, Orjalo A, Park M, Fischer WH, Forbes DJ. Vasu S, et al. J Cell Biol. 2001 Oct 29;155(3):339-54. doi: 10.1083/jcb.200108007. Epub 2001 Oct 29. J Cell Biol. 2001. PMID: 11684705 Free PMC article. - Nuclear envelope and nuclear matrix: interactions and dynamics.
Vlcek S, Dechat T, Foisner R. Vlcek S, et al. Cell Mol Life Sci. 2001 Nov;58(12-13):1758-65. doi: 10.1007/PL00000815. Cell Mol Life Sci. 2001. PMID: 11767745 Free PMC article. Review. - Into the basket and beyond: the journey of mRNA through the nuclear pore complex.
Ashkenazy-Titelman A, Shav-Tal Y, Kehlenbach RH. Ashkenazy-Titelman A, et al. Biochem J. 2020 Jan 17;477(1):23-44. doi: 10.1042/BCJ20190132. Biochem J. 2020. PMID: 31913454 Review.
Cited by
- O-GlcNAc modification of nuclear pore complexes accelerates bidirectional transport.
Yoo TY, Mitchison TJ. Yoo TY, et al. J Cell Biol. 2021 Jul 5;220(7):e202010141. doi: 10.1083/jcb.202010141. J Cell Biol. 2021. PMID: 33909044 Free PMC article. - Nucleoporin TPR Affects C2C12 Myogenic Differentiation via Regulation of Myh4 Expression.
Uhlířová J, Šebestová L, Fišer K, Sieger T, Fišerová J, Hozák P. Uhlířová J, et al. Cells. 2021 May 21;10(6):1271. doi: 10.3390/cells10061271. Cells. 2021. PMID: 34063931 Free PMC article. - Versatility at the nuclear pore complex: lessons learned from the nucleoporin Nup153.
Ball JR, Ullman KS. Ball JR, et al. Chromosoma. 2005 Nov;114(5):319-30. doi: 10.1007/s00412-005-0019-3. Epub 2005 Nov 12. Chromosoma. 2005. PMID: 16133350 Review. - Megator, an essential coiled-coil protein that localizes to the putative spindle matrix during mitosis in Drosophila.
Qi H, Rath U, Wang D, Xu YZ, Ding Y, Zhang W, Blacketer MJ, Paddy MR, Girton J, Johansen J, Johansen KM. Qi H, et al. Mol Biol Cell. 2004 Nov;15(11):4854-65. doi: 10.1091/mbc.e04-07-0579. Epub 2004 Sep 8. Mol Biol Cell. 2004. PMID: 15356261 Free PMC article. - Evolution and diversification of the nuclear pore complex.
Makarov AA, Padilla-Mejia NE, Field MC. Makarov AA, et al. Biochem Soc Trans. 2021 Aug 27;49(4):1601-1619. doi: 10.1042/BST20200570. Biochem Soc Trans. 2021. PMID: 34282823 Free PMC article. Review.
References
- Allen, T.D., J.M. Cronshaw, S. Bagley, E. Kiseleva, and M.W. Goldberg. 2000. The nuclear pore complex: mediator of translocation between nucleus and cytoplasm. J. Cell Sci. 113:1651–1659. - PubMed
- Bangs, P.L., C.A. Sparks, P.R. Odgren, and E.G. Fey. 1996. Product of the oncogene-activating gene Tpr is a phosphorylated protein of the nuclear pore complex. J. Cell. Biochem. 61:48–60. - PubMed
- Beesley, R.C. 1993. Immunocytochemistry: a practical approach. Oxford University Press, Oxford, UK. 266 pp.
- Bodoor, K., S. Shaikh, D. Salina, W.H. Raharjo, R. Bastos, M. Lohka, and B. Burke. 1999. Sequential recruitment of NPC proteins to the nuclear periphery at the end of mitosis. J. Cell Sci. 112:2253–2264. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous