G protein-coupled receptor/arrestin3 modulation of the endocytic machinery - PubMed (original) (raw)
G protein-coupled receptor/arrestin3 modulation of the endocytic machinery
Francesca Santini et al. J Cell Biol. 2002.
Abstract
Nonvisual arrestins (arr) modulate G protein-coupled receptor (GPCR) desensitization and internalization and bind to both clathrin (CL) and AP-2 components of the endocytic coated pit (CP). This raises the possibility that endocytosis of some GPCRs may be a consequence of arr-induced de novo CP formation. To directly test this hypothesis, we examined the behavior of green fluorescent protein (GFP)-arr3 in live cells expressing beta2-adrenergic receptors and fluorescent CL. After agonist stimulation, the diffuse GFP-arr3 signal rapidly became punctate and colocalized virtually completely with preexisting CP spots, demonstrating that activated complexes accumulate in previously formed CPs rather than nucleating new CP formation. After arr3 recruitment, CP appeared larger: electron microscopy analysis revealed an increase in both CP number and in the occurrence of clustered CPs. Mutant arr3 proteins with impaired binding to CL or AP-2 displayed reduced recruitment to CPs, but were still capable of inducing CP clustering. In contrast, though constitutively present in CPs, the COOH-terminal moiety of arr3, which contains CP binding sites but lacks receptor binding, did not induce CP clustering. Together, these results indicate that recruitment of functional arr3-GPCR complexes to CP is necessary to induce clustering. Latrunculin B or 16 degrees C blocked CP rearrangements without affecting arr3 recruitment to CP. These results and earlier studies suggest that discrete CP zones exist on cell surfaces, each capable of supporting adjacent CPs, and that the cortical actin membrane skeleton is intimately involved with both the maintenance of existing CPs and the generation of new structures.
Figures
Figure 1.
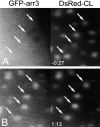
Agonist activation induces recruitment of arr3 to preexisting CPs. HEK293 cells coexpressing GFP-arr3, DsRed-CL, and Flag-tagged β2-AR were viewed as described in Materials and methods. A is frame 1 of Video 1 and shows the distribution of DsRed-CL and GFP-arr3 27 s before addition of ISO (10 μM) to the cells. B is frame 20 of Video 1, acquired 73 s after stimulation and shows the redistribution of GFP-arr3 to preexisting CPs (arrows). Video 1 is available at
http://www.jcb.org/cgi/content/full/jcb.200110132/DC1
.
Figure 2.
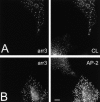
Changes in CP appearance upon β2-AR activation. Following treatment with ISO, COS1 cells coexpressing Flag–β2-AR and arr3 show recruitment of arr3 to CPs, increase in CP size, and increased CP AP-2 signal in comparison to arr3-nonexpressing cells. (A) COS1 cells transiently expressing Flag–β2-AR and arr3 were stimulated with ISO (10 μM, 5 min), fixed, and stained for CL and arr3. (B) COS1 cells transiently expressing Flag–β2-AR and arr3 were stained for AP-2 and arr3 after stimulation with ISO (10 μM, 5 min). Bar, 10 μm.
Figure 3.
Changes in plasma membrane CPs in RBL cells following β2-AR stimulation. RBL(β2) cells stably expressing Flag–β2-AR show formation of larger CPs with increased AP-2 signal as well as recruitment of endogenous arr3 (arr3) to CPs after stimulation with ISO (ISO) (10 μM, 5 min). Insets show fourfold magnified views of the boxed region of the cells. Bar, 10 μm.
Figure 4.
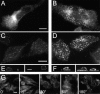
m1AChR stimulation induces changes in plasma membrane CPs. Stimulation of resting RBL(m1) cells (A) with CBC (1 mM, 3 min) induces the formation of larger CPs (B) as revealed by immunostaining for CL. Confocal microscopy images of untreated (C) or CBC-treated cells (D) immunostained for AP-2 also reveals the appearance of larger CPs with greatly increased AP-2 signal. These CPs are appropriately restricted to the plasma membrane (x-z sections; E and F). The confocal images were acquired and reproduced using identical gain settings and the same minimum and maximum display values for each unstimulated/stimulated pair. (G) Lower magnification views of changes in AP-2–labeled CPs over a 3 min period following stimulation. Bars: 10 μm.
Figure 5.
Ultrastructural analysis reveals increased plasma membrane CP clustering in CBC-stimulated RBL(m1) cells. Compared with unstimulated cells (A), agonist stimulation (1 mM, 3 min) produced an increase in the occurrence of nearby or adjacent CPs (B–I). These clusters, which often comprised multiple distinct membrane-coated regions, included pits at different stages of invagination (B–F, and H). See text for details. Bar, 0.2 μm.
Figure 6.
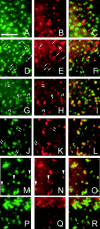
Endogenous arr3 is often concentrated in one CP within a cluster and CP clusters, but not arr3, persist after antagonist treatment. RBL(m1) cells were stimulated with CBC (1 mM) for 0 (A–C), 0.5 (D–F), 1 (G–I), 3 (J–L), or 6 min (M–O). At zero time (A–C), endogenous arr3 (red) was not coincident with CPs, marked by AP-2 (green). Some coincidence of the two signals is evident within 30 s of CBC addition (D–F, arrows). Between 1–3 min after agonist treatment, arr3 is often localized within one of two adjacent CPs (G–L, arrows), less resolvable at later times (M–O). Note that the degree of CP clustering does not correlate closely with intensity of arr3 signal (M–O, arrowheads). P–R show cells treated for 3 min with CBC (1 mM) followed by the antagonist atropine (10 μM) for 3 min. Under these conditions, arr3 has been virtually completely removed from the CPs but the clustered CP phenotype remains unchanged. Bar, 3 μm.
Figure 7.
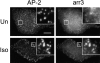
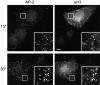
Changes in CP morphology and distribution follow arr3 recruitment to CPs. Recruitment of arr3 to CPs (AP-2) can be visualized after only 15 s (15”) of ISO (10 μM) stimulation in COS1 cells transiently expressing β2-AR and arr3. At this time, the appearance of CPs in the arr3-expressing cells is indistinguishable from that in nonexpressing counterparts. Subsequent to arr3 recruitment (30”), increases in CP size and in AP-2 signal become apparent. Note that there is little proportionality between the levels of arr3 and the AP-2 spot size and brightness (see arrowheads in insets). Insets are fourfold magnified view of the cell area boxed. Bar, 10 μm.
Figure 8.
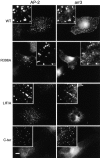
Clustering of CPs requires recruitment of functional arr3. COS1 cells expressing Flag–β2-AR and either wild-type arr3 (WT), arr3 with impaired AP-2- (R396A) or CL-binding (LIF/A), or arr3 284–409 (C-ter) were stimulated with ISO (10 μM, 5 min). Although the extent to which either of the mutant full-length arr3 proteins was recruited to CPs (AP-2, left) was greatly diminished, each was capable of inducing formation of the larger, clustered CPs. However, the COOH-terminal domain of arr3, which colocalized with AP-2 in the absence (unpublished data) or presence (C-ter) of agonist, was incapable of inducing this change in CP appearance. Insets show fourfold magnification of the boxed area of the cell. Bar, 10 μm.
Figure 9.
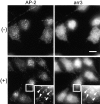
Latrunculin B inhibits agonist-induced CP clustering. RBL(m1) cells were treated with latrunculin B for 3 min before treatment for 5 min with vehicle (−) or CBC (+). Cells were fixed, permeabilized, and immunostained for AP-2 and arr3. Latrunculin B did not affect CP appearance (AP-2) in unstimulated cells (−) nor did it inhibit agonist-induced arr3 recruitment to CPs (+, insets, arrows). However, it did block CP clustering and the rise in CP-associated AP-2. Insets are fourfold magnified views of the cell area boxed. Bar, 10 μm.
Similar articles
- Selective recruitment of arrestin-3 to clathrin coated pits upon stimulation of G protein-coupled receptors.
Santini F, Penn RB, Gagnon AW, Benovic JL, Keen JH. Santini F, et al. J Cell Sci. 2000 Jul;113 ( Pt 13):2463-70. doi: 10.1242/jcs.113.13.2463. J Cell Sci. 2000. PMID: 10852825 - Phosphoinositide 3-kinase regulates beta2-adrenergic receptor endocytosis by AP-2 recruitment to the receptor/beta-arrestin complex.
Naga Prasad SV, Laporte SA, Chamberlain D, Caron MG, Barak L, Rockman HA. Naga Prasad SV, et al. J Cell Biol. 2002 Aug 5;158(3):563-75. doi: 10.1083/jcb.200202113. Epub 2002 Aug 5. J Cell Biol. 2002. PMID: 12163475 Free PMC article. - Beta-arrestin acts as a clathrin adaptor in endocytosis of the beta2-adrenergic receptor.
Goodman OB Jr, Krupnick JG, Santini F, Gurevich VV, Penn RB, Gagnon AW, Keen JH, Benovic JL. Goodman OB Jr, et al. Nature. 1996 Oct 3;383(6599):447-50. doi: 10.1038/383447a0. Nature. 1996. PMID: 8837779 - G-protein coupled receptor kinases as modulators of G-protein signalling.
Bünemann M, Hosey MM. Bünemann M, et al. J Physiol. 1999 May 15;517 ( Pt 1)(Pt 1):5-23. doi: 10.1111/j.1469-7793.1999.0005z.x. J Physiol. 1999. PMID: 10226145 Free PMC article. Review.
Cited by
- Role of Clathrin and Dynamin in Clathrin Mediated Endocytosis/Synaptic Vesicle Recycling and Implications in Neurological Diseases.
Prichard KL, O'Brien NS, Murcia SR, Baker JR, McCluskey A. Prichard KL, et al. Front Cell Neurosci. 2022 Jan 18;15:754110. doi: 10.3389/fncel.2021.754110. eCollection 2021. Front Cell Neurosci. 2022. PMID: 35115907 Free PMC article. Review. - Arrestin domain-containing protein 3 recruits the NEDD4 E3 ligase to mediate ubiquitination of the beta2-adrenergic receptor.
Nabhan JF, Pan H, Lu Q. Nabhan JF, et al. EMBO Rep. 2010 Aug;11(8):605-11. doi: 10.1038/embor.2010.80. Epub 2010 Jun 18. EMBO Rep. 2010. PMID: 20559325 Free PMC article. - Regulation of G protein-coupled receptor signaling by plasma membrane organization and endocytosis.
Weinberg ZY, Puthenveedu MA. Weinberg ZY, et al. Traffic. 2019 Feb;20(2):121-129. doi: 10.1111/tra.12628. Epub 2019 Jan 11. Traffic. 2019. PMID: 30536564 Free PMC article. Review. - CPG2: a brain- and synapse-specific protein that regulates the endocytosis of glutamate receptors.
Cottrell JR, Borok E, Horvath TL, Nedivi E. Cottrell JR, et al. Neuron. 2004 Nov 18;44(4):677-90. doi: 10.1016/j.neuron.2004.10.025. Neuron. 2004. PMID: 15541315 Free PMC article. - Endocytic trafficking of activated EGFR is AP-2 dependent and occurs through preformed clathrin spots.
Rappoport JZ, Simon SM. Rappoport JZ, et al. J Cell Sci. 2009 May 1;122(Pt 9):1301-5. doi: 10.1242/jcs.040030. Epub 2009 Apr 7. J Cell Sci. 2009. PMID: 19351721 Free PMC article.
References
- Ali, H., J.R. Cunha-Melo, W.F. Saul, and M.A. Beaven. 1990. Activation of phospholipase C via adenosine receptors provides synergistic signals for secretion in antigen-stimulated RBL-2H3 cells. Evidence for a novel adenosine receptor. J. Biol. Chem. 265:745–753. - PubMed
- Amoui, M., P. Draber, and L. Draberova. 1997. Src family-selective tyrosine kinase inhibitor, PP1, inhibits both Fc epsilonRI- and Thy-1-mediated activation of rat basophilic leukemia cells. Eur. J. Immunol. 27:1881–1886. - PubMed
- Apgar, J.R. 1990. Antigen-induced cross-linking of the IgE receptor leads to an association with the detergent-insoluble membrane skeleton of rat basophilic leukemia (RBL-2H3) cells. J. Immunol. 145:3814–3822. - PubMed
- Attramadal, H., J.L. Arriza, C. Aoki, T.M. Dawson, J. Codina, M.M. Kwatra, S.H. Snyder, M.G. Caron, and R.J. Lefkowitz. 1992. Beta-arrestin2, a novel member of the arrestin/beta-arrestin gene family. J. Biol. Chem. 267:17882–17890. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous