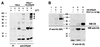ERp44, a novel endoplasmic reticulum folding assistant of the thioredoxin family - PubMed (original) (raw)
ERp44, a novel endoplasmic reticulum folding assistant of the thioredoxin family
Tiziana Anelli et al. EMBO J. 2002.
Abstract
In human cells, Ero1-Lalpha and -Lbeta (hEROs) regulate oxidative protein folding by selectively oxidizing protein disulfide isomerase. Specific protein--protein interactions are probably crucial for regulating the formation, isomerization and reduction of disulfide bonds in the endoplasmic reticulum (ER). To identify molecules involved in ER redox control, we searched for proteins interacting with Ero1-Lalpha. Here, we characterize a novel ER resident protein (ERp44), which contains a thioredoxin domain with a CRFS motif and is induced during ER stress. ERp44 forms mixed disulfides with both hEROs and cargo folding intermediates. Whilst the interaction with transport-competent Ig-K chains is transient, ERp44 binds more stably with J chains, which are retained in the ER and eventually degraded by proteasomes. ERp44 does not bind a short-lived ribophorin mutant lacking cysteines. Its overexpression alters the equilibrium of the different Ero1-Lalpha redox isoforms, suggesting that ERp44 may be involved in the control of oxidative protein folding.
Figures
Fig. 1. Characterization of a novel protein forming mixed disulfides with Ero1-Lα. (A) Anti-myc immunoprecipitates from 107 HeLa cells stably transfected with a vector driving the expression of myc-tagged Ero1-Lα (Ero1-Lα) or with an empty vector (MOCK) were resolved by SDS–PAGE under non-reducing conditions, and stained with Coomassie Blue colloidal. Bands were excised and analysed by MS. The band denoted by an asterisk was shown to contain peptides derived from Ero1-Lα and from an open reading frame, corresponding to KIAA0573, subsequently resequenced from HeLa and SK-N-BE cDNAs. The faint band indicated by a dot was excised and was shown by MS to contain the same peptides as were found in the band indicated by the asterisk. (B) Sequence of the 44 kDa protein forming mixed disulfides with Ero1-Lα. The two peptides sequenced in the mixed disulfide with Ero1-Lα are underlined. Note the presence of a N-terminal hydrophobic sequence (shaded), which is absent in the mature protein, and an RDEL motif at the C-terminus (underlined). The six cysteine residues are indicated by dots. The trx-like domain is boxed. The CRFS motif has a black background. (C) Chromosomal organization of the gene encoding ERp44 in humans. Note the presence of an ERSE-like sequence upstream of the translation initiation site.
Fig. 2. Evolutionary conservation of ERp44. The sequences of ERp44-related polypeptides from M.musculus (18 day embryo cDNA, accession No. AK003217), D.melanogaster (alt1, accession No. AE003501), C.elegans (hypothetical protein C30H7.2, accession No. T25574). The alignment was performed using the pileup algorithm of the Wisconsin Package version 9.0, Genetics Computer Group (GCG; Madison, WI). Black boxes indicate identity and grey similarity.
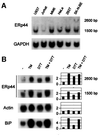
Fig. 3. ERp44 transcripts are widely expressed in human cell lines and can be further induced by ER stress. (A) Northern blot analyses were performed on total RNA extracted from the cell lines indicated, obtained from ATCC. Blots were hybridized sequentially with probes specific for ERp44 (top panel) or GAPDH (bottom panel) for normalization. (B) ERp44 transcripts accumulate during the UPR. SK-N-BE neuroblastoma cells were treated with tunicamycin (TM, 10 µg/ml), DTT (2 mM) or both drugs for 6 h (Benedetti et al., 2000). Blots were hybridized with probes specific for ERp44, actin and BiP, as indicated. Densitometric quantitations are reported on the right, and expressed as fold induction relative to untreated cells.
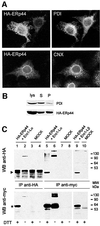
Fig. 4. ERp44 is a soluble ER resident protein forming mixed disulfides with both Ero1-Lα and Ero1-Lβ. (A) ERp44 colocalizes with ER markers. HeLa cells transfected with a plasmid driving the expression of HA–ERp44 were simultaneously stained with anti-HA (left panels, HA–ERp44) and either anti-PDI or anti-calnexin (CNX) and analysed by immunofluorescence. (B) Membrane partitioning of HA–ERp44. Soluble (S) and pelleted (P) proteins were resolved by SDS–PAGE under reducing conditions and transferred to nitrocellulose filters, which were hybridized with anti-PDI (top) or anti-HA–ERp44 (bottom) antibodies. (C) ERp44 forms mixed disulfides with both Ero1-Lα and Ero1-Lβ. HeLa cells were cotransfected with plasmids driving the expression of HA–ERp44 and either Ero1-Lα–myc or Ero1-Lβ–myc, or with empty vectors (MOCK). Lysates were immunoprecipitated with anti-HA or anti-myc. Blots were labelled with the antibodies indicated. The arrowhead and the asterisk indicate monomeric ERp44 and hEROs, respectively. The difference in intensity of the signal in lanes 1 and 2 (reduced and non-reduced, respectively) might be due to the formation of high molecular weight complexes and/or to technical artefacts such as different electrotransfer or epitope accessibility. The faster-migrating band stained by anti-myc (lane 5, bottom panel) might represent unglycosylated, untranslocated or proteolytically cleaved Ero1-Lα, as it is also stained by D5 antibodies (Benham et al., 2000).
Fig. 5. ERp44-containing mixed disulfides in HeLa cells. (A) Lysates of HeLa cells, before (lanes 1–4) or 48 h after transfection with a plasmid driving the expression of HA–ERp44 (lanes 5 and 6), were resolved under reducing (+DTT) or non-reducing (–) conditions and transferred to a nitrocellulose filter, which was labelled with a polyclonal rabbit anti-GST–ERp44 antiserum (lanes 3–6) or with pre-immune serum (PI, lanes 1 and 2) as a control. The lysates of 1.5 × 105 untransfected HeLa cells and of 1 × 105 HA–ERp44-expressing cells were loaded. The different electrophoretic mobilities of the bands indicated by asterisks and dots indicate that both endogenous (lanes 3 and 4, dots) and exogenous ERp44 (lanes 5 and 6, asterisks) form intra-chain disulfide bonds. The presence of the HA tag in exogenous molecules explains their slower mobility with respect to endogenous ERp44. Arrowheads on the right hand margin point to ERp44-containing mixed disulfides. Whilst more abundant in transfected cells, these are detectable also in untransfected HeLa cells (lane 4). The band of ∼26 kDa labelled by the GST–ERp44-specific antiserum might be endogenous GST. (B) Lysates of HeLa cells transfected as indicated were immunoprecipitated with immobilized anti-HA antibodies. Complexes were resolved under reducing (R) or non-reducing (NR) conditions and transferred to nitrocellulose. The blot shown on the right was cut horizontally and the upper and lower panels stained with D5 and anti-HA antibodies, respectively. Complexes including ERp44 and Ero1-Lα (endogenous or transfected) are indicated by §. Only one-third of the immunoprecipitated material was loaded on HA–ERp44+Ero1-Lα lanes.
Fig. 6. ERp44 interacts with partially folded Ig subunits. HeLa cells were cotransfected with plasmids driving the expression of HA–ERp44 and myc-tagged Ig-K or J chains (K or JcM, respectively) or of a truncated ribophorin mutant (Ri332). (A) Transport-competent K chains interact transiently with ERp44. HeLa cells co-expressing HA–ERp44 and K chains were pulsed for 5 min with 35S-labelled amino acids in the presence of 3 mM DTT and chased for the times indicated without the reducing agent. Lysates were immunoprecipitated with anti-HA and resolved under non-reducing (NR, upper panel) or reducing (R, lower panel) conditions. The mobility of monomeric and ERp44-bonded K is indicated on the right hand margin. (B) JcM folding intermediates interact stably with ERp44. HeLa cells co-expressing HA–ERp44 and JcM were pulse–chased, immunopre cipitated and resolved as in (A). The bands indicated by the bracket are precipitated by anti-HA also from K-expressing cells (see A) and might correspond to endogenous ERp44 substrates. (C) A short-lived mutant ribophorin that lacks cysteine residues (Ri332) does not interact with ERp44. Lysates from HeLa cells co-expressing HA–ERp44 and Ri332 were immunoprecipitated with anti-HA. The immunoprecipitated material (IP) and 1/20 of that left over (LO) after immunoprecipitation were loaded on to the gel. Blots were hybridized with anti-HA or anti-ribophorin antibodies, as indicated.
Fig. 7. Functional role of ERp44 in oxidative protein folding. (A) DTT and diamide alter the distribution of the different redox isoforms of Ero1-Lα (OX1 and OX2). HeLa cells expressing myc-tagged Ero1-Lα were treated for 5 min with 5 mM DTT or diamide (DIA) before lysis and electrophoresis under NR conditions. Western blots were labelled with anti-myc antibodies. (B) ERp44 overexpression favours the accumulation of OX2. HeLa cells expressing myc-tagged Ero1-Lα alone or with HA–ERp44 were analysed by western blotting as in (A). Dots indicate bands containing only Ero1-Lα and ERp44, while the asterisk indicates Ero1-Lα and PDI complex. The ‘smile’ generated by lateral diffusion of the reducing agent between adjacent lanes underscores that OX1 and OX2 are redox isoforms. (C) OX1/OX2 ratios were calculated by densitometry in HeLa transfectants expressing Ero1-Lα alone or with HA–ERp44. The average of four experiments and standard deviation are shown. The OX1/OX2 ratio changed slightly in different experiments depending on cell concentration and method of extraction. However, it was consistently lower in cells overexpressing ERp44 than in matched controls.
Similar articles
- JPDI, a novel endoplasmic reticulum-resident protein containing both a BiP-interacting J-domain and thioredoxin-like motifs.
Hosoda A, Kimata Y, Tsuru A, Kohno K. Hosoda A, et al. J Biol Chem. 2003 Jan 24;278(4):2669-76. doi: 10.1074/jbc.M208346200. Epub 2002 Nov 20. J Biol Chem. 2003. PMID: 12446677 - The role of ERp44 in maturation of serotonin transporter protein.
Freyaldenhoven S, Li Y, Kocabas AM, Ziu E, Ucer S, Ramanagoudr-Bhojappa R, Miller GP, Kilic F. Freyaldenhoven S, et al. J Biol Chem. 2012 May 18;287(21):17801-17811. doi: 10.1074/jbc.M112.345058. Epub 2012 Mar 26. J Biol Chem. 2012. PMID: 22451649 Free PMC article. Retracted. - Thiol-mediated protein retention in the endoplasmic reticulum: the role of ERp44.
Anelli T, Alessio M, Bachi A, Bergamelli L, Bertoli G, Camerini S, Mezghrani A, Ruffato E, Simmen T, Sitia R. Anelli T, et al. EMBO J. 2003 Oct 1;22(19):5015-22. doi: 10.1093/emboj/cdg491. EMBO J. 2003. PMID: 14517240 Free PMC article. - The physiological functions of mammalian endoplasmic oxidoreductin 1: on disulfides and more.
Ramming T, Appenzeller-Herzog C. Ramming T, et al. Antioxid Redox Signal. 2012 May 15;16(10):1109-18. doi: 10.1089/ars.2011.4475. Epub 2012 Feb 15. Antioxid Redox Signal. 2012. PMID: 22220984 Review. - Proteostasis and "redoxtasis" in the secretory pathway: Tales of tails from ERp44 and immunoglobulins.
Anelli T, Sannino S, Sitia R. Anelli T, et al. Free Radic Biol Med. 2015 Jun;83:323-30. doi: 10.1016/j.freeradbiomed.2015.02.020. Epub 2015 Mar 2. Free Radic Biol Med. 2015. PMID: 25744412 Review.
Cited by
- Role of the early secretory pathway in SARS-CoV-2 infection.
Sicari D, Chatziioannou A, Koutsandreas T, Sitia R, Chevet E. Sicari D, et al. J Cell Biol. 2020 Sep 7;219(9):e202006005. doi: 10.1083/jcb.202006005. J Cell Biol. 2020. PMID: 32725137 Free PMC article. Review. - Division of labor among oxidoreductases: TMX1 preferentially acts on transmembrane polypeptides.
Pisoni GB, Ruddock LW, Bulleid N, Molinari M. Pisoni GB, et al. Mol Biol Cell. 2015 Oct 1;26(19):3390-400. doi: 10.1091/mbc.E15-05-0321. Epub 2015 Aug 5. Mol Biol Cell. 2015. PMID: 26246604 Free PMC article. - VDAC1 regulates neuronal cell loss after retinal trauma injury by a mitochondria-independent pathway.
de Sousa E, Móvio MI, de Lima-Vasconcellos TH, Dos Santos GB, Dos Santos Gomes T, Walter LT, da Silva DA, Rodrigues T, Cerchiaro G, Kihara AH. de Sousa E, et al. Cell Death Dis. 2022 Apr 21;13(4):393. doi: 10.1038/s41419-022-04755-3. Cell Death Dis. 2022. PMID: 35449127 Free PMC article. - The human protein disulphide isomerase family: substrate interactions and functional properties.
Ellgaard L, Ruddock LW. Ellgaard L, et al. EMBO Rep. 2005 Jan;6(1):28-32. doi: 10.1038/sj.embor.7400311. EMBO Rep. 2005. PMID: 15643448 Free PMC article. Review. - Disulfide bonds in ER protein folding and homeostasis.
Feige MJ, Hendershot LM. Feige MJ, et al. Curr Opin Cell Biol. 2011 Apr;23(2):167-75. doi: 10.1016/j.ceb.2010.10.012. Epub 2010 Dec 7. Curr Opin Cell Biol. 2011. PMID: 21144725 Free PMC article. Review.
References
- Alberini C.M., Bet,P., Milstein,C. and Sitia,R. (1990) Secretion of immunoglobulin M assembly intermediates in the presence of reducing agents. Nature, 347, 485–487. - PubMed
- Andres D.A., Dickerson,I.M. and Dixon,J.E. (1990) Variants of the carboxyl-terminal KDEL sequence direct intracellular retention. J. Biol. Chem., 265, 5952–5955. - PubMed
- Benedetti C., Fabbri,M., Sitia,R. and Cabibbo,A. (2000) Aspects of gene regulation during the UPR in human cells. Biochem. Biophys. Res. Commun., 278, 530–536. - PubMed
- Bulleid N.J. and Freedman,R.B. (1988) Defective co-translational formation of disulphide bonds in protein disulphide-isomerase-deficient microsomes. Nature, 335, 649–651. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous