Nuclear pore complex is able to transport macromolecules with diameters of about 39 nm - PubMed (original) (raw)
Nuclear pore complex is able to transport macromolecules with diameters of about 39 nm
Nelly Panté et al. Mol Biol Cell. 2002 Feb.
Abstract
Bidirectional transport of macromolecules between the nucleus and the cytoplasm occurs through the nuclear pore complexes (NPCs) by a signal-mediated mechanism that is directed by targeting signals (NLSs) residing on the transported molecules or "cargoes." Nuclear transport starts after interaction of the targeting signal with soluble cellular receptors. After the formation of the cargo-receptor complex in the cytosol, this complex crosses the NPC. Herein, we use gold particles of various sizes coated with cargo-receptor complexes to determine precisely how large macromolecules crossing the NPC by the signal-mediated transport mechanism could be. We found that cargo-receptor-gold complexes with diameter close to 39 nm could be translocated by the NPC. This implies that macromolecules much larger than the assumed functional NPC diameter of 26 nm can be transported into the karyoplasm. The physiological relevance of this finding was supported by the observation that intact nucleocapsids of human hepatitis B virus with diameters of 32 and 36 nm are able to cross the nuclear pore without disassembly.
Figures
Figure 1
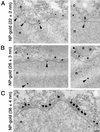
Electron microscopy of representative gold particles and their protein coat. Gold particles (A, 22 nm; B, 26 nm; and C, 36 nm) uncoated (first row) and coated with NP or BSA (second row) were visualized in the electron microscope by negative staining. The third and fourth rows are examples of NP- and BSA-coated gold particles that were incubated first with importin α (NP + α and BSA + α) and then with importin β (NP + α + β and BSA + α + β). The last row shows examples of NP- and BSA-coated gold particles that were incubated with importin α and β and then immunogold labeled with an anti-importin β antibody directly conjugated with 8-nm gold particles.
Figure 2
Nuclear import of NP-coated gold particles into Xenopus oocyte nuclei. Gold particles of 22 ± 2 nm (A), 26 ± 3 nm (B), and 36 ± 4 nm (C) in diameter were coated with NP and their nuclear import was followed by electron microscopy 1 h after injection into the cytoplasm of Xenopus oocytes. Shown are views of nuclear envelope cross sections with adjacent cytoplasm (c) and nucleoplasm (n). Arrowheads point to gold particles that reached the nucleus or are associated with the nuclear face of the NPC. Gold particles 28 nm in diameter or smaller (without the protein coat) were imported into the nucleus. Bar, 100 nm.
Figure 3
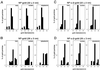
Histograms on the size distribution of gold particles available for import (total), and found within the cytoplasm and the nucleus. Twenty-two ± 2 nm-gold particles, coated with NP (A), 26 ± 3 nm-gold particles, coated with NP (B), 22 ± 2 nm-gold particles, coated with NP and importin α and β (C), and 26 ± 3 nm-gold particles, coated with NP and importin α and β (D). For comparison, only 100 particles in the nucleus and 100 particles in the cytoplasm were measured. The data were derived from microinjections in four different oocytes.
Figure 4
Nuclear import of NP-importin α- and β-coated gold particles into Xenopus oocyte nuclei. NP-coated gold particles with diameter of 26 ± 3 nm were incubated in vitro first with importin α and then with importin β. The resulting complexes were injected into the cytoplasm of Xenopus oocytes and their nuclear import was followed by electron microscopy. Shown are low- (A) and high (B)-magnification views of nuclear envelope cross sections with adjacent cytoplasm (c) and nucleoplasm (n). Arrowheads point to gold particles that reached the nucleus or are associated with the nuclear face of the NPC. Gold particles as large as 26 nm in diameter (without the protein coat) were able to cross the nuclear pores and enter the nucleus of Xenopus oocytes. Shown is also a gallery of gold particles that were found in the nucleus of Xenopus oocytes. Bars, 200 nm (A) and 50 nm (B).
Figure 5
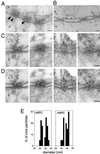
Intact hepatitis B virus cores (32 or 36 nm in diameter) are able to cross the nuclear pores. (A) Views of a nuclear envelope cross section with adjacent cytoplasm (c) and nucleoplasm (n) from a Xenopus oocyte that have had phosphorylated recombinant HBV cores microinjected into their cytoplasm. Arrowheads point to core particles associated with the nuclear face of the NPC. (B) Nuclear envelope cross section from a control (noninjected) Xenopus oocyte. (C) Examples of cross-sectioned NPCs with associated HBV cores. (D) Same micrographs as in C but with the nuclear membrane boundaries and the HVB cores outlined. (E) Quantitative analysis of the size of core particles associated with the cytoplasmic face of the nuclear pore (cNPC) or the nuclear face of the nuclear pore (nNPC) from oocytes injected with recombinant HBV cores. 60 HBV cores were measured for each histogram. Bars, 100 nm.
Similar articles
- Dynamic nuclear pore complexes: life on the edge.
Tran EJ, Wente SR. Tran EJ, et al. Cell. 2006 Jun 16;125(6):1041-53. doi: 10.1016/j.cell.2006.05.027. Cell. 2006. PMID: 16777596 Review. - Nuclear transport of single molecules: dwell times at the nuclear pore complex.
Kubitscheck U, Grünwald D, Hoekstra A, Rohleder D, Kues T, Siebrasse JP, Peters R. Kubitscheck U, et al. J Cell Biol. 2005 Jan 17;168(2):233-43. doi: 10.1083/jcb.200411005. J Cell Biol. 2005. PMID: 15657394 Free PMC article. - Activation of ryanodine receptors in the nuclear envelope alters the conformation of the nuclear pore complex.
Erickson ES, Mooren OL, Moore-Nichols D, Dunn RC. Erickson ES, et al. Biophys Chem. 2004 Dec 1;112(1):1-7. doi: 10.1016/j.bpc.2004.06.010. Biophys Chem. 2004. PMID: 15501570 - Exceptional structural and mechanical flexibility of the nuclear pore complex.
Liashkovich I, Meyring A, Kramer A, Shahin V. Liashkovich I, et al. J Cell Physiol. 2011 Mar;226(3):675-82. doi: 10.1002/jcp.22382. J Cell Physiol. 2011. PMID: 20717933 - Nuclear localization signals and human disease.
McLane LM, Corbett AH. McLane LM, et al. IUBMB Life. 2009 Jul;61(7):697-706. doi: 10.1002/iub.194. IUBMB Life. 2009. PMID: 19514019 Review.
Cited by
- Off to the organelles - killing cancer cells with targeted gold nanoparticles.
Kodiha M, Wang YM, Hutter E, Maysinger D, Stochaj U. Kodiha M, et al. Theranostics. 2015 Jan 21;5(4):357-70. doi: 10.7150/thno.10657. eCollection 2015. Theranostics. 2015. PMID: 25699096 Free PMC article. Review. - HIV-1 capsid: the multifaceted key player in HIV-1 infection.
Campbell EM, Hope TJ. Campbell EM, et al. Nat Rev Microbiol. 2015 Aug;13(8):471-83. doi: 10.1038/nrmicro3503. Nat Rev Microbiol. 2015. PMID: 26179359 Free PMC article. Review. - Autoantigens of the nuclear pore complex.
Enarson P, Rattner JB, Ou Y, Miyachi K, Horigome T, Fritzler MJ. Enarson P, et al. J Mol Med (Berl). 2004 Jul;82(7):423-33. doi: 10.1007/s00109-004-0554-z. Epub 2004 Jun 3. J Mol Med (Berl). 2004. PMID: 15175862 Review. - 'Shared-Hook' and 'Changed-Hook' Binding Activities of Herpesviral Core Nuclear Egress Complexes Identified by Random Mutagenesis.
Lösing J, Häge S, Schütz M, Wagner S, Wardin J, Sticht H, Marschall M. Lösing J, et al. Cells. 2022 Dec 13;11(24):4030. doi: 10.3390/cells11244030. Cells. 2022. PMID: 36552794 Free PMC article. - Impact of the Nuclear Envelope on Malignant Transformation, Motility, and Survival of Lung Cancer Cells.
Stefanello ST, Luchtefeld I, Liashkovich I, Pethö Z, Azzam I, Bulk E, Rosso G, Döhlinger L, Hesse B, Oeckinghaus A, Shahin V. Stefanello ST, et al. Adv Sci (Weinh). 2021 Nov;8(22):e2102757. doi: 10.1002/advs.202102757. Epub 2021 Oct 17. Adv Sci (Weinh). 2021. PMID: 34658143 Free PMC article.
References
- Baschong W, Wrigley NG. Small colloidal gold conjugated to Fab fragments or to immunoglobulin G as high-resolution labels for electron microscopy: a technical overview. J Electron Microsc Tech. 1990;14:313–323. - PubMed
- Catimel B, Teh T, Fontes MR, Jennings IG, Jans DA, Howlett GJ, Nice EC, Kobe B. Biophysical characterization of interactions involving importin-alpha during nuclear import. J Biol Chem. 2001;276:34189–34198. - PubMed
- Chook YM, Cingolani G, Conti E, Stewart M, Vetter I, Wittinghofer A. Pictures in cell biology. Structures of nuclear-transport components. Trends Cell Biol. 1999;9:310–311. - PubMed
- Cingolani G, Petosa C, Weis K, Muller CW. Structure of importin-beta bound to the IBB domain of importin-alpha. Nature. 1999;399:221–229. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources