The multiprotein exocyst complex is essential for cell separation in Schizosaccharomyces pombe - PubMed (original) (raw)
The multiprotein exocyst complex is essential for cell separation in Schizosaccharomyces pombe
Hongyan Wang et al. Mol Biol Cell. 2002 Feb.
Abstract
Schizosaccharomyces pombe cells divide by medial fission through the use of an actomyosin-based contractile ring. A mulitlayered division septum is assembled in concert with ring constriction. Finally, cleavage of the inner layer of the division septum results in the liberation of daughter cells. Although numerous studies have focused on actomyosin ring and division septum assembly, little information is available on the mechanism of cell separation. Here we describe a mutant, sec8-1, that is defective in cell separation but not in other aspects of cytokinesis. sec8-1 mutants accumulate about 100-nm vesicles and have reduced secretion of acid phosphatase, suggesting that they are defective in exocytosis. Sec8p is a component of the exocyst complex. Using biochemical methods, we show that Sec8p physically interacts with other members of the exocyst complex, including Sec6p, Sec10p, and Exo70p. These exocyst proteins localize to regions of active exocytosis-at the growing ends of interphase cells and in the medial region of cells undergoing cytokinesis-in an F-actin-dependent and exocytosis-independent manner. Analysis of a number of mutations in various exocyst components has established that these components are essential for cell viability. Interestingly, all exocyst mutants analyzed appear to be able to elongate and to assemble division septa but are defective for cell separation. We therefore propose that the fission yeast exocyst is involved in targeting of enzymes responsible for septum cleavage. We further propose that cell elongation and division septum assembly can continue with minimal levels of exocyst function.
Figures
Figure 1
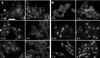
Phenotype of mut2-1 (sec8-1) cells. (A) Growth curves of wild-type and mut2-1 cells. Cell numbers of wild-type and mut2-1 cells were quantified by counting cells using a hemacytometer after temperature shift from 24 to 36°C. ●, wild-type; ○, mut2-1. (B) mut2-1 is defective in cell separation. mut2-1 cells were grown at 24°C to log phase and shifted to the restrictive temperature of 36°C. Samples were collected just before the temperature shift (0 h) and 4 h later and stained with 4′,6-diamidino-2-phenylindole (DAPI) and rhodamine-conjugated phalloidin to visualize DNA and F-actin (top and middle panels) or with Calcofluor to visualize septa (bottom panels). A constricting actomyosin ring is shown with an arrow. Bar: 10 μm.
Figure 2
S. pombe Sec8p is homologous to proteins from S. cerevisiae and human. Amino acid sequences were aligned using ClustalX and Boxshader programs. Identical amino acids are shaded in black, and conservative substitutions are shaded in gray. Hs, Homo sapiens; Sc, S. cerevisiae; Sp, S. pombe.
Figure 3
sec8-1 cells are not defective in polarized cell growth. Wild-type cells (A) and sec8-1 cells (B) were synchronized in G1 by growth in nitrogen-free medium for 18 h at 24°C, and shifted to 36°C for 1 h to inactivate the Sec8-1p protein. Cells were then resuspended in rich medium to allow cell cycle progression at 36°C. Samples were collected just before resuspending in rich medium (0 h) and at intervals thereafter and stained with Calcofluor to visualize septa. Bar: 10 μm.
Figure 4
Alignment of S. pombe Sec6p, Sec10p, Sec15p, and Exo70p with related proteins from S. cerevisiae, rat, and human. Amino acid sequences are aligned using ClustalX and Boxshader programs. Identical amino acids are shaded in black, and conservative substitutions are shaded in gray. Rn, Rattus norvegicus; Hs, Homo sapiens; Sp, S. pombe; Sc, S. cerevisiae.
Figure 5
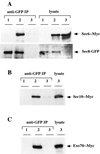
Sec6p, Sec8p, Sec10p, and Exo70p associate in vivo. (A) Protein extracts were prepared from cells expressing Sec8-GFP (lanes 1), Sec8-GFP and Sec6-Myc (lanes 2), or Sec6-Myc alone (lanes 3). Total lysates (right panel) and immunoprecipitates prepared using anti-GFP antibodies (left panel) were blotted using anti-Myc (upper panels) and anti-GFP antibodies (lower panels). (B and C) Similar experiments were conducted using cells expressing Sec8-GFP, Sec10-Myc and Sec8-GFP, or Sec10-Myc (B) or cells expressing Sec8-GFP, Exo70-Myc and Sec8-GFP, or Exo70-Myc (C).
Figure 6
Localization of exocyst components in S. pombe. (A) Exocyst components localize to the division site and cell tips. _sec8-GFP sec6_-Myc cells were stained with antibodies against GFP and Myc. In the merged micrograph, Sec8-GFP is in red, Sec6-Myc in green, and DNA in blue. Arrowheads: tip localization; 1, 4: a single ring structure; 2, 3: double ring structures. (B) Sec8-GFP appeared as two rings in late mitotic cells using confocal microscopy and 3D viewing. Top panel, cell with 0° rotation; lower panel, cell with 139° rotation. (C and D) Merged micrographs of Sec8-GFP with Sec10-Myc (C) and Exo70-Myc (D). Sec8-GFP is in red, Sec10-Myc or Exo70-Myc in green, and DNA in blue. (E) The localization of Sec10p in relation to Myo2p, an actomyosin ring marker. _cdc25_-22 cells expressing Sec10p-GFP were synchronized in G2 by incubation at 36°C for 4 h and allowed to enter mitosis by shifting to 24°C. Cells were stained with antibodies against GFP and Myo2p. (F) The localization of Sec10p is dependent on intact F-actin structures. _cdc25_-22 cells expressing Sec10p-GFP weretreated with either LatA in DMSO (bottom panels) or DMSO alone (top panels) upon release into mitosis. Samples were stained with antibodies against GFP and α-tubulin to visualize Sec10-GFP and microtubules. (G and H) Localization of Sec10p to the division site is lost in cdc12-112 (G) and cdc15-140 (H) mutants. Cells were grown at 24°C, shifted to 36°C for 4 h, and stained with antibodies against GFP and α-tubulin to visualize Sec10-GFP and microtubules. (I) The localization of Sec10p is insensitive to brefeldin A treatment. _cdc25_-22 cells expressing Sec10-GFP or Gma12-GFP (as a marker for the Golgi) were treated with either brefeldin A in ethanol (bottom panels) or ethanol alone (top panels) upon release into mitosis. Samples were stained with DAPI and with antibodies against GFP to visualize Sec10-GFP or Gma12-GFP and DNA. Bars: (A–D) 10 μm; (E–I) 5 μm.
Figure 7
(A, D, and E) Null mutants of sec8 (A), sec6 (D), and sec10 (E) are defective in cleavage of the septum. Mutant spores were germinated in appropriate selective media and stained with DAPI, phalloidin, and Calcofluor to visualize DNA, F-actin, and septa, respectively. (B) Germinated _sec8_-null mutant cells do not contain detectable maternal Sec8p (see text for details). (C) sec8 shut-off cells are defective in cell separation. _81nmt1_-sec8 cells were grown in thiamine-containing medium for 14 h at 30°C and stained with DAPI, phalloidin, and Calcofluor to visualize DNA, F-actin, and septa, respectively. Bar: 10 μm.
Figure 8
sec8 mutants are defective in exocytosis. (A-D) sec8-1 mutant and sec8 shut-off cells accumulate large numbers of secretory vesicles. (A and B) Wild-type (A1, A2) and sec8-1 (B1–B3) mutant cells were grown at 24°C and shifted to 36°C for 4 h, fixed, and processed for electron microscopic analysis. Higher magnifications of the framed regions in A1 and B1 are also shown (A1′ and B1′). Arrows point at presumed secretory vesicles; arrowheads point at division septa. (C and D) sec8 shut-off cells were grown in medium lacking (C) or containing (D) thiamine at 30°C, fixed, and processed for electron microscopic analysis. Note that in the mutant cell forming a septum (D), vesicles were accumulated in the vicinity of the septum. The average number of vesicles in wild-type (5 cells were quantified) and sec8-1 (6 cells were quantified) were 1 and 53, respectively. Bars: 0.5 μm in the two magnified boxes; 1 μm in all other cells. (E) sec8-1 secretes less activity of acid phosphatase. Wild-type (squares) and sec8-1 (triangles) cells were assayed for secreted acid phosphatase activity at 24°C (open) and 36°C (filled).
Similar articles
- Rho3p regulates cell separation by modulating exocyst function in Schizosaccharomyces pombe.
Wang H, Tang X, Balasubramanian MK. Wang H, et al. Genetics. 2003 Aug;164(4):1323-31. doi: 10.1093/genetics/164.4.1323. Genetics. 2003. PMID: 12930742 Free PMC article. - Rng2p, a protein required for cytokinesis in fission yeast, is a component of the actomyosin ring and the spindle pole body.
Eng K, Naqvi NI, Wong KC, Balasubramanian MK. Eng K, et al. Curr Biol. 1998 May 21;8(11):611-21. doi: 10.1016/s0960-9822(98)70248-9. Curr Biol. 1998. PMID: 9635188 - Fission yeast Rng3p: an UCS-domain protein that mediates myosin II assembly during cytokinesis.
Wong KC, Naqvi NI, Iino Y, Yamamoto M, Balasubramanian MK. Wong KC, et al. J Cell Sci. 2000 Jul;113 ( Pt 13):2421-32. doi: 10.1242/jcs.113.13.2421. J Cell Sci. 2000. PMID: 10852821 - The price of independence: cell separation in fission yeast.
Martín-García R, Santos B. Martín-García R, et al. World J Microbiol Biotechnol. 2016 Apr;32(4):65. doi: 10.1007/s11274-016-2021-8. Epub 2016 Mar 1. World J Microbiol Biotechnol. 2016. PMID: 26931605 Review. - Overview of fission yeast septation.
Pérez P, Cortés JC, Martín-García R, Ribas JC. Pérez P, et al. Cell Microbiol. 2016 Sep;18(9):1201-7. doi: 10.1111/cmi.12611. Epub 2016 Jun 1. Cell Microbiol. 2016. PMID: 27155541 Review.
Cited by
- Spontaneous Cdc42 polarization independent of GDI-mediated extraction and actin-based trafficking.
Bendezú FO, Vincenzetti V, Vavylonis D, Wyss R, Vogel H, Martin SG. Bendezú FO, et al. PLoS Biol. 2015 Apr 2;13(4):e1002097. doi: 10.1371/journal.pbio.1002097. eCollection 2015 Apr. PLoS Biol. 2015. PMID: 25837586 Free PMC article. - Adaptation of core mechanisms to generate cell polarity.
Nelson WJ. Nelson WJ. Nature. 2003 Apr 17;422(6933):766-74. doi: 10.1038/nature01602. Nature. 2003. PMID: 12700771 Free PMC article. Review. - Sec5, a member of the exocyst complex, mediates Drosophila embryo cellularization.
Murthy M, Teodoro RO, Miller TP, Schwarz TL. Murthy M, et al. Development. 2010 Aug;137(16):2773-83. doi: 10.1242/dev.048330. Epub 2010 Jul 14. Development. 2010. PMID: 20630948 Free PMC article. - Sip1, an AP-1 accessory protein in fission yeast, is required for localization of Rho3 GTPase.
Yu Y, Li C, Kita A, Katayama Y, Kubouchi K, Udo M, Imanaka Y, Ueda S, Masuko T, Sugiura R. Yu Y, et al. PLoS One. 2013 Jul 1;8(7):e68488. doi: 10.1371/journal.pone.0068488. Print 2013. PLoS One. 2013. PMID: 23840894 Free PMC article. - The exocytic Rabs Ypt3 and Ypt2 regulate the early step of biogenesis of the spore plasma membrane in fission yeast.
Imada K, Nakamura T. Imada K, et al. Mol Biol Cell. 2016 Nov 1;27(21):3317-3328. doi: 10.1091/mbc.E16-03-0162. Epub 2016 Sep 14. Mol Biol Cell. 2016. PMID: 27630265 Free PMC article.
References
- Bähler J, Wu J-Q, Longtine MS, Shah NG, McKenzie A, Steever AB, Wach A, Philippsen P, Pringle JR. Heterologous modules for efficient and versatile PCR-based gene targeting in Schizosaccharomyces pombe. Yeast. 1998b;14:943–951. - PubMed
- Balasubramanian MK, McCollum D, Gould KL. Cytokinesis in fission yeast Schizosaccharomyces pombe. Methods Enzymol. 1997;283:494–506. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases