Cooperativity of Nkx3.1 and Pten loss of function in a mouse model of prostate carcinogenesis - PubMed (original) (raw)
Cooperativity of Nkx3.1 and Pten loss of function in a mouse model of prostate carcinogenesis
Minjung J Kim et al. Proc Natl Acad Sci U S A. 2002.
Abstract
Mouse models have provided significant insights into the molecular mechanisms of tumor suppressor gene function. Here we use mouse models of prostate carcinogenesis to demonstrate that the Nkx3.1 homeobox gene undergoes epigenetic inactivation through loss of protein expression. Loss of function of Nkx3.1 in mice cooperates with loss of function of the Pten tumor suppressor gene in cancer progression. This cooperativity results in the synergistic activation of Akt (protein kinase B), a key modulator of cell growth and survival. Our findings underscore the significance of interactions between tissue-specific regulators such as Nkx3.1 and broad-spectrum tumor suppressors such as Pten in contributing to the distinct phenotypes of different cancers.
Figures
Figure 1
HGPIN/early carcinoma lesions in_Nkx3.1_;Pten compound mutant prostates. Hematoxylin and eosin staining of anterior prostates of_Nkx3.1_;Pten compound mutants at 6 months is shown. (A and B) Well differentiated columnar epithelium. (Inset) High-power view. (C and D) Localized region of severely dysplastic epithelium (arrows) surrounded by well differentiated columnar epithelium. (Inset) Example of nuclear atypia. (E and F) Moderately hyperplastic epithelium. (G and H) HGPIN/early carcinoma lesion (arrow) surrounded by relatively unaffected epithelium. (I and J) Extensive low-grade PIN (LGPIN, arrows). (Inset) Example of nuclear atypia. (K and L) HGPIN/early carcinoma lesion surrounded by relatively unaffected epithelium. (Inset) Atypical nuclei with mitotic figure. (Scale bars, 100 microns.)
Figure 2
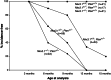
Loss of function of Nkx3.1 and _Pten_cooperate in prostate cancer progression. A graphical representation of the percentage of mice lacking HGPIN/early carcinoma lesions in the dorsolateral prostate at the indicated ages is shown. Incidence corresponds to the occurrence of HGPIN/early carcinoma lesions, which is defined by using the histopathological and morphological criteria described in the text. The percentage incidence-free was calculated by dividing the number of unaffected mice by the total number of mice analyzed for each age group.
Figure 3
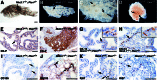
Analysis of HGPIN/early carcinoma lesions. (A–D) Bright-field (A) and dark-field (B–D) images of anterior prostates at 6 months. (A–C) Whole mounts show light dense masses corresponding to the HGPIN/early carcinoma lesions (arrows). (D) Microdissected lesion containing numerous blood vessels (arrows). (Scale bars, 500 microns.) (E–L) Immunohistochemical analysis of the anterior prostate at 6 months. (E and F) Robust staining of wide-spectrum cytokeratins (CK-P) in HGPIN/early carcinoma lesions. (G and H) Immunodetection with anti-CK14 (arrows) shows an absence of basal cells in lesion. (Insets) High-power views. (I and_J_) Anti-CD105 (endoglin) shows increased microvascularization (J, arrows) of lesions. (K and L) Ki67 antibody shows increased cellular proliferation in lesions. (Scale bars, 100 microns.)
Figure 4
Absence of Nkx3.1 protein expression in mouse and human prostate cancer. (A–F) Immunohistochemical analysis of the anterior prostate of Nkx3.1 and Pten_mutants with an anti-mouse Nkx3.1 polyclonal antisera. (A) Uniform immunostaining of luminal epithelium with adjacent stroma unstained (arrow). (Inset) Nuclear staining of secretory cells and absence of staining of basal cells (arrow). (B) Absence of staining in LGPIN in a 12-month prostate. (Inset) Unstained and stained nuclei at the margin of the PIN (arrow). (C and D) Lack of staining in HGPIN in a 6-month prostate (C, arrow) and in an HGPIN/early carcinoma lesion of an 8-month prostate (D, arrow). Note the uniform staining of adjacent unaffected regions (C). (Inset) Unstained nuclei with atypia and mitotic figure (arrow). (E) Heterogeneity of staining (arrows) in a cluster of hyperplastic cells in an 8-month prostate. (Inset) Juxtaposition of stained, unstained, and lightly stained nuclei. (F) Example of cytoplasmic immunostaining (arrows and inset) at the margins of an HGPIN/early carcinoma lesion in a 12-month prostate. (G–I) Immunohistochemical analysis of human prostatectomy specimens using a polyclonal anti-human NKX3.1 antisera. (G) Immunostaining of normal prostate epithelium (NPE). Note absence of staining in basal cells (arrows) and stroma. (Inset) Nuclear staining of secretory cells (arrow). (H) Absent or heterogeneous staining in well differentiated cancer (CaP) compared with adjacent normal prostate epithelium. (Inset) Lack of staining in cancer cells (arrow). (I) Predominantly cytoplasmic immunostaining of a poorly differentiated cancer (arrows and Inset). (J–L) Immunohistochemical analysis of the anterior prostate using anti-Pten antisera. (J) Uniform staining in the epithelium and stroma. (K) Moderate reduction of Pten staining in a region of HGPIN in a 5-month prostate (arrows). (L) Absence of Pten staining in an HGPIN/early carcinoma lesion of a 12-month prostate. Note that in K and L_the staining of adjacent unaffected regions is similar to wild type (J). (M–O) Analysis of allelic status of_Nkx3.1 and Pten in HGPIN/early carcinoma lesions. (M) LCM was performed on Nkx3.1-immunostained sections to isolate genomic DNA from lesions (n = 20, Nkx3.1-nonexpressing) and adjacent unaffected regions (n = 8, Nkx3.1-expressing); representative data are shown. (N and O) Southern blot analysis to detect the wild-type (WT) alleles for_Nkx3.1 and Pten; detection of the targeted allele (KO) serves as an internal control. (P) Reverse transcriptase–PCR analysis showing robust expression of_Nkx3.1_ and reduced expression of Pten in HGPIN/early carcinoma lesion of_Nkx3.1_+/−;Pten+/−mutant relative to the adjacent unaffected epithelium. cDNA was prepared in the absence (−) or presence (+) of reverse transcriptase (RT). Note that Nkx3.1 is not expressed in bladder; glyceraldehyde-3-phosphate dehydrogenase is the positive control.
Figure 5
Synergistic activation of Akt in_Nkx3.1_;Pten compound mutant prostates. (A) Western blot analysis shows high levels of activated Akt in protein extracts from the anterior prostate but not the bladder of Nkx3.1;Pten compound mutants at 8 months. Protein lysates (20 μg) were resolved by SDS/PAGE and probed with antisera to detect P-Akt, total Akt, Pten, or Nkx3.1. Note the reduced levels of Pten in the_Nkx3.1_−/−;Pten+/−compound mutants relative to the Pten single mutants. (B–G) Immunohistochemical analysis of P-Akt in the anterior prostates of Nkx3.1 and _Pten_mutants. (B) Absence of staining in wild-type mice. (C) Staining in a cluster of hyperplastic cells (arrows and Inset). (D) Robust staining of HGPIN/early carcinoma lesion (Inset). (E–G) P-Akt immunostaining in clusters of cells in the_Nkx3.1_−/− prostates shows nuclear and cell surface staining (E, Inset) and primarily nuclear staining (G, Inset).
Similar articles
- Tuberous sclerosis complex 1: an epithelial tumor suppressor essential to prevent spontaneous prostate cancer in aged mice.
Kladney RD, Cardiff RD, Kwiatkowski DJ, Chiang GG, Weber JD, Arbeit JM, Lu ZH. Kladney RD, et al. Cancer Res. 2010 Nov 1;70(21):8937-47. doi: 10.1158/0008-5472.CAN-10-1646. Epub 2010 Oct 12. Cancer Res. 2010. PMID: 20940396 Free PMC article. - Nkx3.1 mutant mice recapitulate early stages of prostate carcinogenesis.
Kim MJ, Bhatia-Gaur R, Banach-Petrosky WA, Desai N, Wang Y, Hayward SW, Cunha GR, Cardiff RD, Shen MM, Abate-Shen C. Kim MJ, et al. Cancer Res. 2002 Jun 1;62(11):2999-3004. Cancer Res. 2002. PMID: 12036903 - Nkx3.1; Pten mutant mice develop invasive prostate adenocarcinoma and lymph node metastases.
Abate-Shen C, Banach-Petrosky WA, Sun X, Economides KD, Desai N, Gregg JP, Borowsky AD, Cardiff RD, Shen MM. Abate-Shen C, et al. Cancer Res. 2003 Jul 15;63(14):3886-90. Cancer Res. 2003. PMID: 12873978 - Pten signaling in gliomas.
Knobbe CB, Merlo A, Reifenberger G. Knobbe CB, et al. Neuro Oncol. 2002 Jul;4(3):196-211. Neuro Oncol. 2002. PMID: 12084351 Free PMC article. Review. - [PTEN expression in endometrial cancer and the prognosis].
Kanamori Y, Uegaki K, Kigawa J, Terakawa N. Kanamori Y, et al. Nihon Rinsho. 2004 Oct;62 Suppl 10:406-9. Nihon Rinsho. 2004. PMID: 15535277 Review. Japanese. No abstract available.
Cited by
- OCT-4: a novel estrogen receptor-α collaborator that promotes tamoxifen resistance in breast cancer cells.
Bhatt S, Stender JD, Joshi S, Wu G, Katzenellenbogen BS. Bhatt S, et al. Oncogene. 2016 Nov 3;35(44):5722-5734. doi: 10.1038/onc.2016.105. Epub 2016 Apr 11. Oncogene. 2016. PMID: 27065334 - Additive Effect of Zfhx3/Atbf1 and Pten Deletion on Mouse Prostatic Tumorigenesis.
Sun X, Xing C, Fu X, Li J, Zhang B, Frierson HF Jr, Dong JT. Sun X, et al. J Genet Genomics. 2015 Jul 20;42(7):373-82. doi: 10.1016/j.jgg.2015.06.004. Epub 2015 Jun 25. J Genet Genomics. 2015. PMID: 26233892 Free PMC article. - Deletion of NKX3.1 via CRISPR/Cas9 Induces Prostatic Intraepithelial Neoplasia in C57BL/6 Mice.
Park JJ, Kim JE, Jeon Y, Lee MR, Choi JY, Song BR, Park JW, Kang MJ, Choi HJ, Bae SJ, Lee H, Kang BC, Hwang DY. Park JJ, et al. Technol Cancer Res Treat. 2020 Jan-Dec;19:1533033820964425. doi: 10.1177/1533033820964425. Technol Cancer Res Treat. 2020. PMID: 33094683 Free PMC article. - Tuberous sclerosis complex 1: an epithelial tumor suppressor essential to prevent spontaneous prostate cancer in aged mice.
Kladney RD, Cardiff RD, Kwiatkowski DJ, Chiang GG, Weber JD, Arbeit JM, Lu ZH. Kladney RD, et al. Cancer Res. 2010 Nov 1;70(21):8937-47. doi: 10.1158/0008-5472.CAN-10-1646. Epub 2010 Oct 12. Cancer Res. 2010. PMID: 20940396 Free PMC article. - Pten (phosphatase and tensin homologue gene) haploinsufficiency promotes insulin hypersensitivity.
Wong JT, Kim PT, Peacock JW, Yau TY, Mui AL, Chung SW, Sossi V, Doudet D, Green D, Ruth TJ, Parsons R, Verchere CB, Ong CJ. Wong JT, et al. Diabetologia. 2007 Feb;50(2):395-403. doi: 10.1007/s00125-006-0531-x. Epub 2006 Dec 29. Diabetologia. 2007. PMID: 17195063 Free PMC article.
References
- Jacks T. Annu Rev Genet. 1996;30:603–636. - PubMed
- Macleod K F, Jacks T. J Pathol. 1999;187:43–60. - PubMed
- Abate-Shen C, Shen M M. Genes Dev. 2000;14:2410–2434. - PubMed
- Matusik R, Masumori M, Thomas T, Case T, Paul M, Kasper S, Shappell S B. In: Transgenics in Endocrinology. Matzuk M, Brown C, Kumar T, editors. Totowa, NJ: Humana; 2002. pp. 401–425.
- Green J E, Greenberg N M, Ashendel C L, Barrett J C, Boone C, Getzenberg R H, Henkin J, Matusik R, Janus T J, Scher H I. Prostate. 1998;36:59–63. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- CA76501/CA/NCI NIH HHS/United States
- R01 DK060887/DK/NIDDK NIH HHS/United States
- R01 CA082783-02/CA/NCI NIH HHS/United States
- U01 CA084294/CA/NCI NIH HHS/United States
- UO1 CA84294/CA/NCI NIH HHS/United States
- DK60887/DK/NIDDK NIH HHS/United States
- R01 CA082783-01A2/CA/NCI NIH HHS/United States
- R01 CA082783/CA/NCI NIH HHS/United States
- R01 CA076501/CA/NCI NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous