Quorum-sensing regulators control virulence gene expression in Vibrio cholerae - PubMed (original) (raw)
Quorum-sensing regulators control virulence gene expression in Vibrio cholerae
Jun Zhu et al. Proc Natl Acad Sci U S A. 2002.
Abstract
The production of virulence factors including cholera toxin and the toxin-coregulated pilus in the human pathogen Vibrio cholerae is strongly influenced by environmental conditions. The well-characterized ToxR signal transduction cascade is responsible for sensing and integrating the environmental information and controlling the virulence regulon. We show here that, in addition to the known components of the ToxR signaling circuit, quorum-sensing regulators are involved in regulation of V. cholerae virulence. We focused on the regulators LuxO and HapR because homologues of these two proteins control quorum sensing in the closely related luminous marine bacterium Vibrio harveyi. Using an infant mouse model, we found that a luxO mutant is severely defective in colonization of the small intestine. Gene arrays were used to profile transcription in the V. cholerae wild type and the luxO mutant. These studies revealed that the ToxR regulon is repressed in the luxO mutant, and that this effect is mediated by another negative regulator, HapR. We show that LuxO represses hapR expression early in log-phase growth, and constitutive expression of hapR blocks ToxR-regulon expression. Additionally, LuxO and HapR regulate a variety of other cellular processes including motility, protease production, and biofilm formation. Together these data suggest a role for quorum sensing in modulating expression of blocks of virulence genes in a reciprocal fashion in vivo.
Figures
Figure 1
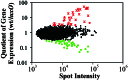
Gene expression profiles for wild-type C6706 and the luxO V. cholerae mutant. Genes with higher expression in the wild-type strain compared with the luxO mutant are shown in red. Genes expressed at higher levels in the luxO mutant compared with the wild-type strain are shown in green. Black dots represent genes that displayed less than 3-fold variation between the two strains. * indicate genes known to be essential for virulence.
Figure 2
CT and TcpA production in wild-type C6706 and mutant V. cholerae strains. Samples were prepared after 5 h incubation in AKI medium with aeration at 37°C. (Upper) Cell pellets from the specified strains were subjected to Western blot and probed with anti-TcpA antibody. (Lower) The corresponding cell-free culture fluids were assayed in GM1 ganglioside enzyme-linked immunosorbent CT assays.
Figure 3
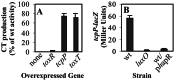
LuxO represses tcpP expression. (A)toxR, tcpP, and toxT were cloned into the expression vector pBAD24 (33), and the plasmids were introduced into the V. cholerae luxO mutant. Subsequently, the strains were grown under AKI-inducing conditions in the presence of 0.01% arabinose. CT production was quantitated after 5 h incubation at 37°C with aeration. The data are presented as the percentage of CT production of the wild-type bearing the same plasmids. (B) tcpP-lacZ expression was assayed in the wild type, the luxO mutant, and the wild-type strain constitutively expressing a cloned hapR_gene (denoted p_hapR). β-galactosidase activity assays (39) were conducted after growth with aeration for 5 h at 37°C in AKI medium.
Figure 4
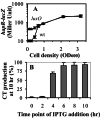
LuxO regulation of hapR expression and HapR regulation of CT production. (A) Wild-type and _luxO_mutants carrying a chromosomal hapR-lacZ reporter fusion were grown in AKI medium at 37°C. Samples were withdrawn at the specified ODs, and β-galactosidase activity was assayed. ●, wild type (wt); ▴,luxO mutant. (B) Wild-type V. cholerae containing the vector pMal-c2x or carrying pJZ146 (pMal-c2X containing hapR under IPTG control) were grown as described in AKI medium, and IPTG (50 μM final concentration) was added at the indicated time points. All of the samples were assayed for CT production after a total of 10 h at 37°C. The data are presented as the percentage of CT production in the V. cholerae strain carrying pJZ146 compared with the strain carrying only the vector pMal-c2x.
Figure 5
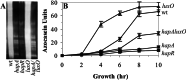
HA protease production in V. cholerae wild-type and mutant strains. (A) Zymogram analyses of cell-free culture fluids prepared from the designated _V. cholerae_parent and isogenic mutant strains after 17 h incubation at 37°C are shown. In lanes prepared from samples containing high HA protease activity [wild type (wt) and luxO_], the HA protease results in complete clearing of the gelatin because the HA protease is active during the electrophoresis run. (B) A time course of HA protease production is shown for the same strains analyzed in_A. Overnight cultures were diluted 1:100 in LB and incubated at 37°C. Samples were taken at 2-h intervals for determination of azocasein activity. One azocasein unit is defined as the amount of enzyme producing an increase of 0.01 OD units per h.
Figure 6
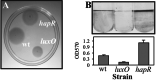
LuxO and HapR control multiple processes in V. cholerae. (A) Different V. cholerae strains were inoculated into motility agar (LB containing 0.3% agar) and incubated at 37°C for 4 h, after which the photograph was taken. (B) A comparison of biofilms produced by wild-type_V. cholerae_ and the luxO and_hapR_ mutants. (Upper) The photograph shows the crystal violet staining in the borosilicate tubes containing the different strains. The normalized data are presented for these assays (Lower). The OD570 values are a measure of crystal-violet staining, which is proportional to the level of biofilm formation. The designation wt refers to the wild-type strain.
Figure 7
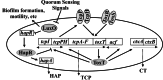
A model for quorum-sensing regulation of V. cholerae_virulence. Solid arrows denote positive effects while solid T bars denote negative effects. At low cell density, LuxO is active and represses the expression of hapR. HapR is a negative regulator of tcpP transcription, so under this condition, the TcpP signaling protein is present and it, together with TcpH and ToxRS, activates the expression of virulence factors TCP and CT. In contrast, at high cell density, LuxO is inactive as a result of autoinducer signal accumulation. Inactivation of LuxO results in_hapR expression at high cell density. HapR represses TcpP and the ToxR regulon and activates Hap protease expression.
Similar articles
- Regulation of virulence gene expression in Vibrio cholerae by quorum sensing: HapR functions at the aphA promoter.
Kovacikova G, Skorupski K. Kovacikova G, et al. Mol Microbiol. 2002 Nov;46(4):1135-47. doi: 10.1046/j.1365-2958.2002.03229.x. Mol Microbiol. 2002. PMID: 12421317 - Vibrio cholerae CsrA Regulates ToxR Levels in Response to Amino Acids and Is Essential for Virulence.
Mey AR, Butz HA, Payne SM. Mey AR, et al. mBio. 2015 Aug 4;6(4):e01064. doi: 10.1128/mBio.01064-15. mBio. 2015. PMID: 26242626 Free PMC article. - The transcriptional regulator VqmA increases expression of the quorum-sensing activator HapR in Vibrio cholerae.
Liu Z, Hsiao A, Joelsson A, Zhu J. Liu Z, et al. J Bacteriol. 2006 Apr;188(7):2446-53. doi: 10.1128/JB.188.7.2446-2453.2006. J Bacteriol. 2006. PMID: 16547031 Free PMC article. - Regulation of virulence in Vibrio cholerae: the ToxR regulon.
Childers BM, Klose KE. Childers BM, et al. Future Microbiol. 2007 Jun;2(3):335-44. doi: 10.2217/17460913.2.3.335. Future Microbiol. 2007. PMID: 17661707 Review. - Quorum Sensing Gene Regulation by LuxR/HapR Master Regulators in Vibrios.
Ball AS, Chaparian RR, van Kessel JC. Ball AS, et al. J Bacteriol. 2017 Sep 5;199(19):e00105-17. doi: 10.1128/JB.00105-17. Print 2017 Oct 1. J Bacteriol. 2017. PMID: 28484045 Free PMC article. Review.
Cited by
- The low-resolution solution structure of Vibrio cholerae Hfq in complex with Qrr1 sRNA.
Vincent HA, Henderson CA, Stone CM, Cary PD, Gowers DM, Sobott F, Taylor JEN, Callaghan AJ. Vincent HA, et al. Nucleic Acids Res. 2012 Sep 1;40(17):8698-710. doi: 10.1093/nar/gks582. Epub 2012 Jun 22. Nucleic Acids Res. 2012. PMID: 22730296 Free PMC article. - Relative contributions of Vibrio polysaccharide and quorum sensing to the resistance of Vibrio cholerae to predation by heterotrophic protists.
Sun S, Kjelleberg S, McDougald D. Sun S, et al. PLoS One. 2013;8(2):e56338. doi: 10.1371/journal.pone.0056338. Epub 2013 Feb 18. PLoS One. 2013. PMID: 23441178 Free PMC article. - Exploiting quorum sensing to confuse bacterial pathogens.
LaSarre B, Federle MJ. LaSarre B, et al. Microbiol Mol Biol Rev. 2013 Mar;77(1):73-111. doi: 10.1128/MMBR.00046-12. Microbiol Mol Biol Rev. 2013. PMID: 23471618 Free PMC article. Review. - Regulation of flagellar motility during biofilm formation.
Guttenplan SB, Kearns DB. Guttenplan SB, et al. FEMS Microbiol Rev. 2013 Nov;37(6):849-71. doi: 10.1111/1574-6976.12018. Epub 2013 Apr 12. FEMS Microbiol Rev. 2013. PMID: 23480406 Free PMC article. Review. - Serogroup conversion of Vibrio cholerae in aquatic reservoirs.
Blokesch M, Schoolnik GK. Blokesch M, et al. PLoS Pathog. 2007 Jun;3(6):e81. doi: 10.1371/journal.ppat.0030081. PLoS Pathog. 2007. PMID: 17559304 Free PMC article.
References
- Lee S H, Hava D L, Waldor M K, Camilli A. Cell. 1999;99:625–634. - PubMed
- Skorupski K, Taylor R K. Mol Microbiol. 1997;25:1003–1009. - PubMed
- Miller M B, Bassler B L. Annu Rev Microbiol. 2001;55:165–199. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases