Transgenesis by lentiviral vectors: lack of gene silencing in mammalian embryonic stem cells and preimplantation embryos - PubMed (original) (raw)
Transgenesis by lentiviral vectors: lack of gene silencing in mammalian embryonic stem cells and preimplantation embryos
Alexander Pfeifer et al. Proc Natl Acad Sci U S A. 2002.
Abstract
The introduction of foreign genes into early mouse embryos and embryonic stem (ES) cells is invaluable for the analysis of gene function and regulation in the living animal. The use of vectors derived from retroviruses as gene transfer vehicles in this setting has had limited success because of silencing of transgene expression. Here, we show that vectors derived from lentiviruses, which are complex retroviruses, can efficiently deliver genes to murine ES cells and that transgene expression is stable during proliferation of undifferentiated ES cells. The transgene is expressed during differentiation of ES cells in vitro (embryoid bodies) and in vivo (teratomas). Transfer of lentivector-transduced ES cells into blastocysts resulted in chimeric animals that expressed the transgene in multiple tissues. Embryos derived from crossings of chimeric mice expressed the transgene, indicating successful germ-line transmission. Infection of murine preimplantation embryos at morula stage with lentiviral vectors resulted in stable transduction and expression of the transgene in mouse embryos and in newborn mice. Finally, human ES cells were transduced by lentiviral vectors and expressed the transgene over several passages. Thus, lentiviral vectors represent a significant improvement over oncoretroviral vectors used previously for gene transfer into murine ES cells and preimplantation embryos. Ability to transfer foreign genes into human ES cells has potential relevance for the development of gene and cell-based therapies.
Figures
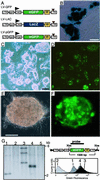
Figure 1
Transduction of mammalian ES cells by using lentiviral vectors. (A) Schematic representation of the lentiviral vectors. (Top and Middle) Lentiviral vectors LV-GFP and LV-LAC contain the compound chicken β-actin/cytomegalovirus enhancer (CAG) promoter, which drives expression of the eGFP or the cDNA for β-galactosidase (LacZ), respectively. (Bottom) LV-pGFP contains the promoter of the PGK. All vectors carry a central polypurine tract of HIV-1 (ppt), a posttranscriptional regulatory element of the woodchuck hepatitis virus (W), and self-inactivating mutations (brown triangle) in the LTR. (B) Expression of LacZ in D3 ES cells transduced with LV-LAC. ES cell colonies were stained with 5-bromo-4-chloro-3-indoyl-β-
d
-galactoside (X-Gal). [Bar = 50 μm.] (C and D) Representative bright field (C) and fluorescence microscopy (D) images of D3 ES cells 2 weeks after infection with LV-GFP (moi 50). [Bar = 200 μm.] (E and F) Transduction of human ES cells with LV-GFP. Bright field (E) and fluorescence (F) images of one representative ES cell colony are shown. [Bar = 100 μm.] (G) Southern blot and flow cytometer analyses of murine ES cell clones. (Left) Representative Southern blot of four ES cell clones (lanes 1–4) derived from D3 ES cells infected with LV-GFP (moi 5) and uninfected ES cells (lane 5). (Right Top) Schematic structure of the integrated lentivirus; wavy lines, mouse chromosome. Genomic ES cell DNA was digested with _Xba_I, which cleaves the provirus only once, blotted, and hybridized to the indicated probe (black bar) to assess provirus copy number. (Right Bottom) Flow cytometry analysis of the same ES cell clones (–4). DNA from ES cell clone in lane 2 is negative for eGFP.
Figure 2
Expression of the lentiviral transgene during in vitro and in vivo differentiation of ES cells. (A_–_D) EBs derived from ES cells transduced with LV-GFP (A and C) vs. EBs derived from uninfected ES cells (B and D). Bright field (A and B) and fluorescent microscopy (C and D) images of the same EB are shown (see Fig. 5: contractile activity of the EB shown in A and C). [Bar = 200 μm.] (E_–_G) Analysis of myosin (E), desmin (F), and neurofilament (NF200) (G) expression in EB-derived cells. Single-channel confocal microscopy analysis was performed (red channel, immunofluorescence stain; green channel, eGFP), and a merged-color image is shown. [Bar = 20 μm.] The asterisks indicate the nucleus, and the arrowheads mark axon-like structures of two NF200-positive cells. (H_–_J) Transgene expression in teratomas derived from lentivirus-transduced ES cells. (H) In vivo imaging analysis of eGFP expression in a mouse injected with LV-GFP-transduced D3 ES cells (arrows). (I_–_L) Histological analysis of teratomas derived from LV-GFP-transduced ES cells (I and J) and control ES cells (K and L). The hematoxylin/eosin stain (I and K) and the fluorescence microscopy (J and L) images of adjacent sections (thickness, 5 μm) are shown. The arrowheads indicate stratified squamous epithelium, whereas the arrows mark muscle. The asterisks indicate epithelial gut-like structures. [Bar = 50 μm.] Nuclear DNA was stained with 4′,6-diamidino-2-phenylindole (DAPI), blue.
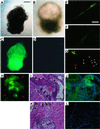
Figure 3
Lentiviral vector-transduced ES cells give rise to chimeras that express the transgene in different tissues. (A) In vivo imaging of green fluorescence in a 3-day-old chimera (no. 1) derived from LV-GFP-transduced ES cells and two nonchimeric animals (nos. 2 and 3). (B_–_G) eGFP expression in the eye (B and E), brain (C and F), and muscle (D and G) of an adult chimeric mouse with ≈40% coat chimerism. Shown are the bright field (B_–_D) and epifluorescence images (E_–_G); the arrowheads and arrows in C and F indicate eGFP-positive skeletal muscle and cerebrum, respectively. (H_–_O) Histological analysis of eGFP expression in the spleen (H and L), cerebellum (I and M), and skeletal muscle (J and N) of a chimeric mouse and skeletal muscle of a control mouse (K and O). The hematoxylin/eosin stain (H_–_K) and the fluorescence microscopy (L_–_O) images of adjacent cryosections are shown. [Bar = 100 μm.] 4′,6-diamidino-2-phenylindole (DAPI) stain, blue. (P) Immunoblot analysis using polyclonal anti-GFP Abs on extracts of muscle (lane 1) and cerebrum (lane 2) of the same chimeric mouse shown in B_–_D. (Q_–_T) Germ-line transmission of the transgene. Fertilized eggs were isolated from superovulated females, and eGFP expression was analyzed by using a confocal microscope. Representative example of an eGFP-positive embryo (4-cell stage) derived from matings of male chimeric mice (Q and R) and eGFP-negative embryos derived from matings of control mice (S and T). [Bar = 100 μm.]
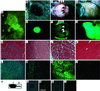
Figure 4
Transduction of murine preimplantation embryos with lentiviral vectors. (A_–_H) eGFP expression in cultured blastocysts derived from morulae infected with LV-GFP. At 96 h after infection (B and F), eGFP is expressed in inner cell mass-derived components (asterisk) and trophoblast-like cells (arrow). Blastocysts derived from morulae that are surrounded by a zona pellucida (arrow in A and E) or from uninfected morulae (C and G) are eGFP-negative. [Bar = 100 μm.] (I_–_L) Transgene expression in an embryo (day 12 postcoitus) derived from lentivirus-infected morulae. Bright field (I and K) and fluorescence (J and L) images of one representative embryo (I and J) and its placenta (K and L) are shown. [Bar = 1 mm.] (M and N) eGFP expression in a 3-day-old mosaic animal derived from an LV-GFP-infected morula; original magnification (×0.2). The bright field (M) and the green fluorescence (N) images are shown. (O) PCR detection of lentiviral vector DNA. PCR analysis of genomic DNA isolated from a transgenic CAG-GFP mouse (1), control mouse (2), empty lane (3), embryo shown in I and J (4), placenta shown in K and L (5), and newborn mosaic mouse shown in M and N (6).
Similar articles
- High levels of transgene expression following transduction of long-term NOD/SCID-repopulating human cells with a modified lentiviral vector.
Gao Z, Golob J, Tanavde VM, Civin CI, Hawley RG, Cheng L. Gao Z, et al. Stem Cells. 2001;19(3):247-59. doi: 10.1634/stemcells.19-3-247. Stem Cells. 2001. PMID: 11359950 - High-level sustained transgene expression in human embryonic stem cells using lentiviral vectors.
Ma Y, Ramezani A, Lewis R, Hawley RG, Thomson JA. Ma Y, et al. Stem Cells. 2003;21(1):111-7. doi: 10.1634/stemcells.21-1-111. Stem Cells. 2003. PMID: 12529558 - Gene transfer in ovarian cancer cells: a comparison between retroviral and lentiviral vectors.
Indraccolo S, Habeler W, Tisato V, Stievano L, Piovan E, Tosello V, Esposito G, Wagner R, Uberla K, Chieco-Bianchi L, Amadori A. Indraccolo S, et al. Cancer Res. 2002 Nov 1;62(21):6099-107. Cancer Res. 2002. PMID: 12414634 - Retroviral vectors for gene therapy of AIDS and cancer.
Chang LJ, He J. Chang LJ, et al. Curr Opin Mol Ther. 2001 Oct;3(5):468-75. Curr Opin Mol Ther. 2001. PMID: 11699891 Review. - Lentiviral vectors: excellent tools for experimental gene transfer and promising candidates for gene therapy.
Vigna E, Naldini L. Vigna E, et al. J Gene Med. 2000 Sep-Oct;2(5):308-16. doi: 10.1002/1521-2254(200009/10)2:5<308::AID-JGM131>3.0.CO;2-3. J Gene Med. 2000. PMID: 11045424 Review.
Cited by
- Lentiviral mediated transgenesis by in vivo manipulation of spermatogonial stem cells.
Sehgal L, Thorat R, Khapare N, Mukhopadhaya A, Sawant M, Dalal SN. Sehgal L, et al. PLoS One. 2011;6(7):e21975. doi: 10.1371/journal.pone.0021975. Epub 2011 Jul 7. PLoS One. 2011. PMID: 21760937 Free PMC article. - Lentiviral Vectors for Ocular Gene Therapy.
Arsenijevic Y, Berger A, Udry F, Kostic C. Arsenijevic Y, et al. Pharmaceutics. 2022 Jul 31;14(8):1605. doi: 10.3390/pharmaceutics14081605. Pharmaceutics. 2022. PMID: 36015231 Free PMC article. Review. - Preliminary study of microRNA-126 as a novel therapeutic target for primary hypertension.
Liu J, Liu J, Shi L, Zhang F, Yu L, Yang X, Cai J. Liu J, et al. Int J Mol Med. 2018 Apr;41(4):1835-1844. doi: 10.3892/ijmm.2018.3420. Epub 2018 Jan 23. Int J Mol Med. 2018. PMID: 29393351 Free PMC article. - Murine retroviruses re-engineered for lineage tracing and expression of toxic genes in the developing chick embryo.
Venters SJ, Dias da Silva MR, Hyer J. Venters SJ, et al. Dev Dyn. 2008 Nov;237(11):3260-9. doi: 10.1002/dvdy.21766. Dev Dyn. 2008. PMID: 18942139 Free PMC article. - Regulator of G-protein signaling-10 negatively regulates NF-κB in microglia and neuroprotects dopaminergic neurons in hemiparkinsonian rats.
Lee JK, Chung J, McAlpine FE, Tansey MG. Lee JK, et al. J Neurosci. 2011 Aug 17;31(33):11879-88. doi: 10.1523/JNEUROSCI.1002-11.2011. J Neurosci. 2011. PMID: 21849548 Free PMC article.
References
- Evans M J, Kaufman M H. Nature (London) 1981;292:154–156. - PubMed
- Robertson E, Bradley A, Kuehn M, Evans M. Nature (London) 1986;323:445–448. - PubMed
- Stewart C L, Ruther U, Garber C, Vanek M, Wagner E F. J Embryol Exp Morphol. 1986;97,Suppl.:263–275. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials