Attenuation of thermal nociception and hyperalgesia by VR1 blockers - PubMed (original) (raw)
. 2002 Feb 19;99(4):2374-9.
doi: 10.1073/pnas.022285899.
Marc Humet, Rosa Planells-Cases, Ana Gomis, Marco Caprini, Felix Viana, Elvira De La Pena, Francisco Sanchez-Baeza, Teresa Carbonell, Carmen De Felipe, Enrique Pérez-Paya, Carlos Belmonte, Angel Messeguer, Antonio Ferrer-Montiel
Affiliations
- PMID: 11854530
- PMCID: PMC122372
- DOI: 10.1073/pnas.022285899
Attenuation of thermal nociception and hyperalgesia by VR1 blockers
Carolina García-Martinez et al. Proc Natl Acad Sci U S A. 2002.
Abstract
Vanilloid receptor subunit 1 (VR1) appears to play a critical role in the transduction of noxious chemical and thermal stimuli by sensory nerve endings in peripheral tissues. Thus, VR1 antagonists are useful compounds to unravel the contribution of this receptor to pain perception, as well as to induce analgesia. We have used a combinatorial approach to identify new, nonpeptidic channel blockers of VR1. Screening of a library of trimers of N-alkylglycines resulted in the identification of two molecules referred to as DD161515 [N-[2-(2-(N-methylpyrrolidinyl)ethyl]glycyl]-[N-[2,4-dichlorophenethyl]glycyl]-N-(2,4-dichlorophenethyl)glycinamide] and DD191515 [[N-[3-(N,N-diethylamino)propyl]glycyl]-[N-[2,4-dichlorophenethyl]glycyl]-N-(2,4-dichlorophenethyl)glycinamide] that selectively block VR1 channel activity with micromolar efficacy, rivaling that characteristic of vanilloid-related inhibitors. These compounds appear to be noncompetitive VR1 antagonists that recognize a receptor site distinct from that of capsaicin. Intraperitoneal administration of both trialkylglycines into mice significantly attenuated thermal nociception as measured in the hot plate test. It is noteworthy that these compounds eliminated pain and neurogenic inflammation evoked by intradermal injection of capsaicin into the animal hindpaw, as well as the thermal hyperalgesia induced by tissue irritation with nitrogen mustard. In contrast, responses to mechanical stimuli were not modified by either compound. Modulation of sensory nerve fibers excitability appears to underlie the peptoid analgesic activity. Collectively, these results indicate that blockade of VR1 activity attenuates chemical and thermal nociception and hyperalgesia, supporting the tenet that this ionotropic receptor contributes to chemical and thermal sensitivity and pain perception in vivo. These trialkylglycine-based, noncompetitive VR1 antagonists may likely be developed into analgesics to treat inflammatory pain.
Figures
Figure 1
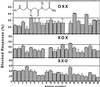
Screening of an oligo N-substituted glycine combinatorial library to identify VR1 channel blockers. VR1 blockade profile of the 66 library mixtures. Each graph represents the blocking activity for each of the three positions that compose the library. The bars denote the activity of each mixture as a function of the number of the defined amine used to generate the chemical diversity. Library mixtures were assayed at 100 μg/ml. Horizontal bar denotes the cut-off blocking activity (set to 50%). (Inset) Generic formula of the trialkylglycine combinatorial library, where R1, R2, and R3 denote the sites where chemical diversity was introduced.
Figure 2
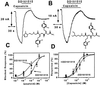
Trialkylglycines are noncompetitive capsaicin antagonists that block VR1 with high efficacy. Representative blockade activity of the trialkylglycines DD161515 (A) and DD191515 (B). Insets depict the chemical structure of both peptoids. VR1 channels were activated by 10 μM capsaicin and recorded at −80 mV. Peptoids were tested at 10 μM. (C) Dose–response curves for compounds DD161515 and DD191515 blockade activity of capsaicin-activated VR1 channels expressed in amphibian oocytes. Responses were normalized with respect to that in the absence of trialkylglycines. Solid lines depict the theoretical fits to a Michaelis–Menten binding isotherm. Each point represents the mean ± SEM with, n ≥ 4. (D) Dose–response curves of VR1 activation by capsaicin in the absence (−DD161515) and presence (+DD161515) of 1 μM peptoid. Data are mean ± SEM, n = 3.
Figure 3
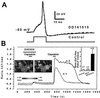
Effect of trialkylglycines on the action potential and capsaicin-evoked [Ca2+]i rises in cultured trigeminal neurons. (A) Action potentials elicited from trigeminal primary neurons in the absence (control) and presence of 10 μM DD161515 in the external solution. Square pulse under the action potential denotes the stimulation protocol under current clamp. (B) The traces show the time course of the fluorescence ratio (F357/F380) changes in the two neurons shown in ratio images (Left Inset) of a culture with trigeminal primary sensory neurons (n1, n2), fibroblasts, and glial cells, during exposure to capsaicin in the presence of D161515 (Left) and after washing out the peptoid (Right). Solid lines depict pulse duration for VR1 agonist and antagonist. (Right Inset) Histogram representing the mean ratio between the second and first [Ca2+]i elevation to capsaicin, in the presence or absence of the indicated compound. Peptoids were tested at 100 μM, and capsaicin was used at 1 μM. Control denotes two consecutive pulses of capsaicin in the absence of peptoid. (Calibration bar of images, 10 μm.)
Figure 4
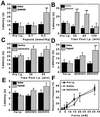
Inhibition by the peptoid of capsaicin-evoked neural activity in knee joint nociceptor fibers. (A_–_D) Instantaneous frequency of the nerve impulse discharge evoked by intraarterial injections of 100 μl capsaicin, 10 μM (arrows) before (A) and 15 min (B), 35 min (C), and 55 min (D) after the administration of 100 μl of a 4 mM (0.4 μmol) solution of the peptoid D161515. (E and F) The impulse discharge elicited by a 10-s knee joint rotation (starting at the arrow) applied before injection of capsaicin and peptoid and 35 min after administration of the peptoid (immediately before C), respectively. (Insets) A sample record of the multiunit impulse activity evoked by capsaicin (A) and by the mechanical stimulation (E).
Figure 5
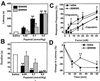
Identified trialkylglycines attenuate thermal nociception. Response latencies in the hot plate at 52°C (A_–_C) and 56°C (D), and the tail immersion at 52°C (E) tests. (F) Response to mechanical stimulation of the hindpaw with von Frey hairs of six intensities. Mice were monitored before (Pre i.p.) and 30 min after i.p. injection of vehicle (saline) or 0.2 mmol/kg of peptoid (unless explicitly indicated in the figure). All data are given as mean ± SEM with n ≥ 9 (hot plate 52°, A_–_C), n ≥ 7 (hot plate 56°C), n ≥ 8 (tail immersion), and n ≥ 9 (von Frey hairs). *, P < 0.05; **, P < 0.01—for saline versus peptoid with the one-way ANOVA test and two tailed-unpaired t test.
Figure 6
Selected trialkylglycines ablate capsaicin-evoked inflammation and thermal hyperalgesia. Latency to first action (A) and duration of action (B) of the behavioral reaction (licking and shaking paw) evoked by intraplantar injection of capsaicin in the absence (saline) or presence of trialkylglycines. (C) Responses to mechanical stimulation of the hindpaw with von Frey hairs. Mice were tested 60 min after i.p. injection of saline or 0.2 mmol/kg DD161515 (−Capsaicin). Thereafter, capsaicin (6 μg) was intradermally administered into one hindpaw of the same animals, which were tested 30 min later (+Capsaicin). (D) Change in hot-plate latency after painting the plantar hindpaw with 10% mustard oil (n = 7). All data are given as mean ± SEM with n ≥ 9 (A and B) or n ≥ 7 (C and D). *, P < 0.05; **, P < 0.01—for saline versus peptoid with the one-way ANOVA test and two tailed-unpaired t test.
Similar articles
- Design and characterization of a noncompetitive antagonist of the transient receptor potential vanilloid subunit 1 channel with in vivo analgesic and anti-inflammatory activity.
García-Martínez C, Fernández-Carvajal A, Valenzuela B, Gomis A, Van Den Nest W, Ferroni S, Carreño C, Belmonte C, Ferrer-Montiel A. García-Martínez C, et al. J Pain. 2006 Oct;7(10):735-46. doi: 10.1016/j.jpain.2006.03.008. J Pain. 2006. PMID: 17018334 - The VR1 antagonist capsazepine reverses mechanical hyperalgesia in models of inflammatory and neuropathic pain.
Walker KM, Urban L, Medhurst SJ, Patel S, Panesar M, Fox AJ, McIntyre P. Walker KM, et al. J Pharmacol Exp Ther. 2003 Jan;304(1):56-62. doi: 10.1124/jpet.102.042010. J Pharmacol Exp Ther. 2003. PMID: 12490575 - Vanilloid receptor ligands: hopes and realities for the future.
Szallasi A. Szallasi A. Drugs Aging. 2001;18(8):561-73. doi: 10.2165/00002512-200118080-00001. Drugs Aging. 2001. PMID: 11587243 Review. - Endovanilloid signaling in pain.
Di Marzo V, Blumberg PM, Szallasi A. Di Marzo V, et al. Curr Opin Neurobiol. 2002 Aug;12(4):372-9. doi: 10.1016/s0959-4388(02)00340-9. Curr Opin Neurobiol. 2002. PMID: 12139983 Review.
Cited by
- Effect of analgesic therapy on clinical outcome measures in a randomized controlled trial using client-owned dogs with hip osteoarthritis.
Malek S, Sample SJ, Schwartz Z, Nemke B, Jacobson PB, Cozzi EM, Schaefer SL, Bleedorn JA, Holzman G, Muir P. Malek S, et al. BMC Vet Res. 2012 Oct 4;8:185. doi: 10.1186/1746-6148-8-185. BMC Vet Res. 2012. PMID: 23035739 Free PMC article. Clinical Trial. - Halogenation of a capsaicin analogue leads to novel vanilloid TRPV1 receptor antagonists.
Appendino G, Harrison S, De Petrocellis L, Daddario N, Bianchi F, Schiano Moriello A, Trevisani M, Benvenuti F, Geppetti P, Di Marzo V. Appendino G, et al. Br J Pharmacol. 2003 Aug;139(8):1417-24. doi: 10.1038/sj.bjp.0705387. Br J Pharmacol. 2003. PMID: 12922928 Free PMC article. - Molecular modeling of the full-length human TRPV1 channel in closed and desensitized states.
Fernández-Ballester G, Ferrer-Montiel A. Fernández-Ballester G, et al. J Membr Biol. 2008 Jun;223(3):161-72. doi: 10.1007/s00232-008-9123-7. Epub 2008 Sep 14. J Membr Biol. 2008. PMID: 18791833 - Immortalization and characterization of a nociceptive dorsal root ganglion sensory neuronal line.
Chen W, Mi R, Haughey N, Oz M, Höke A. Chen W, et al. J Peripher Nerv Syst. 2007 Jun;12(2):121-30. doi: 10.1111/j.1529-8027.2007.00131.x. J Peripher Nerv Syst. 2007. PMID: 17565537 Free PMC article. - Biologic poisons for pain.
Reisner L. Reisner L. Curr Pain Headache Rep. 2004 Dec;8(6):427-34. doi: 10.1007/s11916-004-0063-3. Curr Pain Headache Rep. 2004. PMID: 15509455 Review.
References
- Caterina M J, Schumacher M A, Tominaga M, Rosen T A, Levine J D, Julius D. Nature (London) 1997;389:816–824. - PubMed
- Zygmunt P M, Peterson J, Anderson D A, Chuang H, Sorgard M, Di Marzo V, Julius D, Hogestatt E D. Nature (London) 1999;400:452–457. - PubMed
- Tominaga M, Caterina M J, Malmberg A B, Rosen T A, Gilvert H, Skinner K, Raumann B E, Basbaum A I, Julius D. Neuron. 1998;21:531–543. - PubMed
- Caterina M J, Julius D. Annu Rev. 2001;24:487–517. - PubMed
- Szallasi A, Blumberg P M. Pharmacol Rev. 1999;51:159–211. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical