Weekly cisplatin and daily oral etoposide is highly effective in platinum pretreated ovarian cancer - PubMed (original) (raw)
Weekly cisplatin and daily oral etoposide is highly effective in platinum pretreated ovarian cancer
M E L van der Burg et al. Br J Cancer. 2002.
Free PMC article
Abstract
We investigated the potential of weekly cisplatin and daily oral etoposide followed by oral etoposide maintenance therapy in patients with platinum-refractory ovarium cancer. One hundred and seven patients were entered on the study, 98 patients completed the induction therapy consisting of cisplatin at either 50 or 70 mg m(-2) weekly for six administrations plus oral etoposide at a dose of 50 mg daily. Of these 98 patients, 38 had a platinum treatment-free interval of more than 12 months, 32 had an interval between 4 and 12 months, and 28 had progressed during or within 4 months after last platinum therapy. We assessed response rates and time to progression, and also response duration and survival. Analyses were done on the 98 evaluable patients. All 107 patients were considered evaluable for toxicity. Of the 38 patients with a treatment-free interval of more than 12 months, 92% responded, with 63% complete responses. The median progression-free survival in these patients was 14 months, and the median survival was 26 months. Of the 32 patients with an interval of 4-12 months, 91% responded, with 31% complete responses, a median progression-free interval of 8 and a median overall survival of 16 months. Of the 28 patients with platinum-refractory disease, 46% as yet responded, with 29% complete responses, median progression-free interval of 5 and an overall survival of 13 months. Haematologic and non-haematologic, particularly renal toxicity and neurotoxicity, were notably mild. We conclude that this intensive regimen of weekly cisplatin plus daily etoposide is highly effective and well tolerated in patients with ovarian cancer relapsing after conventional platinum-based combination chemotherapy, including patients who have progressed during or within 4 months after platinum treatment.
Figures
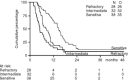
Figure 1
PFS according to platinum sensitivity.
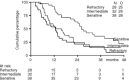
Figure 2
Survival according to platinum sensitivity.
Comment in
- Weekly platinum chemotherapy for recurrent ovarian cancer.
Clamp A, Jayson GC. Clamp A, et al. Br J Cancer. 2002 Jan 7;86(1):2-4. doi: 10.1038/sj.bjc.6600062. Br J Cancer. 2002. PMID: 11857002 Free PMC article.
Similar articles
- Phase II study of prolonged oral etoposide in patients with ovarian cancer refractory to or relapsing within 12 months after platinum-containing chemotherapy.
de Wit R, van der Burg ME, van den Gaast A, Logmans A, Stoter G, Verweij J. de Wit R, et al. Ann Oncol. 1994 Sep;5(7):656-7. doi: 10.1093/oxfordjournals.annonc.a058942. Ann Oncol. 1994. PMID: 7993845 Clinical Trial. - Weekly cisplatin and oral etoposide as treatment for relapsed epithelial ovarian cancer.
Meyer T, Nelstrop AE, Mahmoudi M, Rustin GJ. Meyer T, et al. Ann Oncol. 2001 Dec;12(12):1705-9. doi: 10.1023/a:1013558501425. Ann Oncol. 2001. PMID: 11843248 - Weekly cisplatin with oral etoposide: a well-tolerated and highly effective regimen in relapsed ovarian cancer.
Verborg WA, Campbell LR, Highley MS, Rankin EM. Verborg WA, et al. Int J Gynecol Cancer. 2008 Mar-Apr;18(2):228-34. doi: 10.1111/j.1525-1438.2007.00994.x. Epub 2007 May 19. Int J Gynecol Cancer. 2008. PMID: 17511798 - What is the role of dose-dense therapy?
van der Burg ME, van der Gaast A, Vergote I, Burger CW, van Doorn HC, de Wit R, Stoter G, Verweij J. van der Burg ME, et al. Int J Gynecol Cancer. 2005 Nov-Dec;15 Suppl 3:233-40. doi: 10.1111/j.1525-1438.2005.00432.x. Int J Gynecol Cancer. 2005. PMID: 16343238 Review. - A systematic overview of chemotherapy effects in ovarian cancer.
Högberg T, Glimelius B, Nygren P; SBU-group. Swedish Council of Technology Assessment in Health Care. Högberg T, et al. Acta Oncol. 2001;40(2-3):340-60. doi: 10.1080/02841860151116420. Acta Oncol. 2001. PMID: 11441940 Review.
Cited by
- Outcome of ATP-based tumor chemosensitivity assay directed chemotherapy in heavily pre-treated recurrent ovarian carcinoma.
Sharma S, Neale MH, Di Nicolantonio F, Knight LA, Whitehouse PA, Mercer SJ, Higgins BR, Lamont A, Osborne R, Hindley AC, Kurbacher CM, Cree IA. Sharma S, et al. BMC Cancer. 2003 Jul 3;3:19. doi: 10.1186/1471-2407-3-19. BMC Cancer. 2003. PMID: 12841853 Free PMC article. - Factors favouring long-term survival following recurrence in ovarian cancer.
Soyama H, Takano M, Miyamoto M, Yoshikawa T, Aoyama T, Goto T, Hirata J, Suzuki A, Sasa H, Furuya K. Soyama H, et al. Mol Clin Oncol. 2017 Jul;7(1):42-46. doi: 10.3892/mco.2017.1266. Epub 2017 May 17. Mol Clin Oncol. 2017. PMID: 28685073 Free PMC article. - Combination of irinotecan and platinum for platinum-resistant or refractory recurrent ovarian cancers: A preliminary case series.
Shibutani T, Takano M, Miyamoto M, Yoshikawa T, Aoyama T, Soyama H, Hirata J, Suzuki A, Sasa H, Furuya K. Shibutani T, et al. Mol Clin Oncol. 2017 Jul;7(1):51-55. doi: 10.3892/mco.2017.1258. Epub 2017 May 12. Mol Clin Oncol. 2017. PMID: 28685075 Free PMC article. - Prospective Validation of an Ex Vivo, Patient-Derived 3D Spheroid Model for Response Predictions in Newly Diagnosed Ovarian Cancer.
Shuford S, Wilhelm C, Rayner M, Elrod A, Millard M, Mattingly C, Lotstein A, Smith AM, Guo QJ, O'Donnell L, Elder J, Puls L, Weroha SJ, Hou X, Zanfagnin V, Nick A, Stany MP, Maxwell GL, Conrads T, Sood AK, Orr D, Holmes LM, Gevaert M, Crosswell HE, DesRochers TM. Shuford S, et al. Sci Rep. 2019 Aug 1;9(1):11153. doi: 10.1038/s41598-019-47578-7. Sci Rep. 2019. PMID: 31371750 Free PMC article. - DNA Double-Strand Break Repair Inhibitors: YU238259, A12B4C3 and DDRI-18 Overcome the Cisplatin Resistance in Human Ovarian Cancer Cells, but Not under Hypoxia Conditions.
Macieja A, Gulbas I, Popławski T. Macieja A, et al. Curr Issues Mol Biol. 2023 Sep 28;45(10):7915-7932. doi: 10.3390/cimb45100500. Curr Issues Mol Biol. 2023. PMID: 37886943 Free PMC article.
References
- BerekJSBertelsenKdu BoisABradyMFCarmichaelJEisenhauerEAGoreMGrenmanSHamiltonTCHansenSWHarperPGHorvathGKayeSBLuckHJLundBMcGuireWPNeijtJPOzolsRFParmarMKPiccart-GebhartMJvan RijswijkRRosenbergPRustinGJSessaCWillemsePH1998Advanced epithelial ovarian cancer: 1998 consensus statement Ann Oncol 10S87S92
- BolisGScarfoneGLuchiniLFerrarisCZanaboniFPrestiMGiardinaGVillaAParazziniF1994Response to second-line weekly cisplatin chemotherapy in ovarian cancer previously treated and with a cisplatin or carboplatin-based regimen Eur J Cancer 30A17641768 - PubMed
- ChambersSKChambersJTKohornEISchwartzPE1987Etoposide plus cis-diamminedichloroplatinum as salvage therapy in advanced epithelial ovarian cancer Gynecol Oncol 27233240 - PubMed
- de WitRvan der BurgMELvan der GaastALogmansAStoterGVerweijJ1994Phase II study of prolonged oral etoposide in patients with ovarian cancer refractory to or relapsing within 12 months after platinum containing chemotherapy Ann Oncol 5656657 - PubMed
- EisenhauerEAten Bokkel HuininkWSwenertonKDGianniLMylesJvan der BurgMEKerrIVermorkenJBBuserKColomboN1994European-Canadian randomised trial of Taxol in relapsed ovarian cancer: High vs low and long vs short infusion J Clin Oncol 1226542666 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical