The Rab27a/granuphilin complex regulates the exocytosis of insulin-containing dense-core granules - PubMed (original) (raw)
The Rab27a/granuphilin complex regulates the exocytosis of insulin-containing dense-core granules
Zhaohong Yi et al. Mol Cell Biol. 2002 Mar.
Abstract
Recently, we identified and characterized a novel protein, granuphilin, whose domain structure is similar to that of the Rab3 effector protein rabphilin3 (J. Wang, T. Takeuchi, H. Yokota, and T. Izumi, J. Biol. Chem. 274:28542-28548, 1999). Screening its possible Rab partner by a yeast two-hybrid system revealed that an amino-terminal zinc-finger domain of granuphilin interacts with Rab27a. Granuphilin preferentially bound to the GTP form of Rab27a. Formation of the Rab27a/granuphilin complex was readily detected in the pancreatic beta cell line MIN6. Moreover, the tissue distributions of Rab27a and granuphilin are remarkably similar: both had significant and specific expression in pancreatic islets and in pituitary tissue, but no expression was noted in the brain. Analyses by immunofluorescence, immunoelectron microscopy, and sucrose density gradient subcellular fractionation showed that Rab27a and granuphilin are localized on the membrane of insulin granules. These findings suggest that granuphilin functions as a Rab27a effector protein in beta cells. Overexpression of wild-type Rab27a and its GTPase-deficient mutant significantly enhanced high K(+)-induced insulin secretion without affecting basal insulin release. Although Rab3a, another exocytotic Rab protein, has some similarities with Rab27a in primary sequence, intracellular distribution, and affinity toward granuphilin, overexpression of Rab3a caused different effects on insulin secretion. These results indicate that Rab27a is involved in the regulated exocytosis of conventional dense-core granules possibly through the interaction with granuphilin, in addition to its recently identified role in lysosome-related organelles.
Figures
FIG. 1.
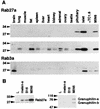
Complex formation between granuphilin and Rab27a. (A) Guanine nucleotide was exchanged on glutathione beads containing GST-fused wild-type Rab27a in buffer (50 mM Tris-HCl [pH 7.5], 5 mM EDTA, 2 mM MgCl2, 150 mM NaCl, and 0.5 mg of bovine serum albumin/ml) with a 1,000-fold molar excess of either GTPγS or GDP at room temperature for 20 min. After the exchange reaction, MgCl2 was added to a final concentration of 7 mM. Aliquots of beads containing either GST alone (lane 1) or GST-fused Rab27a preloaded with GDP (lane 2) or GTPγS (lane 3) were incubated on ice for 30 min with HA-granuphilin-a that had been translated in vitro using the TNT coupled reticulocyte lysate system (Promega, Madison, Wis.). After washing, granuphilin associated with the beads was detected by immunoblotting using anti-HA antibodies. (B) Extracts of MIN6 cells were incubated with either anti-Rab27a (lane 1) or anti-Rab3a (lane 2) antibodies and then with protein G-Sepharose beads. After the beads were washed, immunoprecipitates were analyzed by immunoblotting using antigranuphilin antibodies (αGrp-aC). Although a Rab3a/granuphilin complex is not visible in this figure, eventually a faint band was observed after longer exposure (data not shown). (C) MIN6 cell extracts (lanes 1 and 4), Rab27a (lane 2) and Rab3a (lane 5) immunoprecipitates as prepared for panel B, and anti-Rab27a (lane 3) and anti-Rab3a (lane 6) antibodies themselves were loaded onto an 18% polyacrylamide gel without dithiothreitol to keep most immunoglobulin G-derived proteins off the 20- to 40-kDa range of gels. Immunoblotting was performed using anti-Rab27a (lanes 1 to 3) and anti-Rab3a (lanes 4 to 6) antibodies. Note that each immunoprecipitate contained a significant amount of corresponding Rab protein (asterisks). Rab3a migrated slower than did a 27-kDa molecular mass marker on nonreducing gels. Numbers to the left of each panel are molecular masses in kilodaltons.
FIG. 2.
Tissue and cell expression of Rab27a, Rab3a, and granuphilin. (A) Isolation of mouse pancreatic islets with collagenase and preparation of mouse tissues and cell extracts were performed as described previously (37). An equal amount of protein (100 μg) was loaded onto a polyacrylamide gel. Immunoblotting was performed using anti-Rab27a (top panel) and anti-Rab3a (bottom panel) antibodies. A longer exposure of the Rab27a immunoblot is also shown (middle panel). (B) An equal amount of protein (30 μg) from mouse cell lines (L fibroblast, melan-a melanocyte, B16 melanoma, and MIN6 cells) was loaded onto a polyacrylamide gel. Immunoblotting was performed using anti-Rab27a (left panel) and antigranuphilin (αGrp-N, right panel) antibodies. Numbers to the left of each panel are molecular masses in kilodaltons.
FIG. 3.
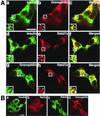
Intracellular distribution of granuphilin and Rab27a. (A) MIN6 cells were double immunostained with antibodies against insulin (a and d), granuphilin (b and g), and Rab27a (e and h) and examined by confocal microscopy. Merged fluorescent signals are shown in subpanels c, f, and i. Insets represent higher-magnification micrographs of cells within the regions outlined by frames. Images represent single confocal sections. Bar, 10 μm. (B) MIN6 cells were similarly double immunostained with antibodies against insulin, Rab3a, and granuphilin. Bar, 10 μm. (C) Ultrathin cryosections of mouse pancreatic beta cells were immunolabeled with rabbit antibodies against granuphilin (αGrp-N, left panel, 5-nm gold particles) and Rab27a (right panel, 10-nm gold particles). Bar, 500 nm.
FIG. 3.
Intracellular distribution of granuphilin and Rab27a. (A) MIN6 cells were double immunostained with antibodies against insulin (a and d), granuphilin (b and g), and Rab27a (e and h) and examined by confocal microscopy. Merged fluorescent signals are shown in subpanels c, f, and i. Insets represent higher-magnification micrographs of cells within the regions outlined by frames. Images represent single confocal sections. Bar, 10 μm. (B) MIN6 cells were similarly double immunostained with antibodies against insulin, Rab3a, and granuphilin. Bar, 10 μm. (C) Ultrathin cryosections of mouse pancreatic beta cells were immunolabeled with rabbit antibodies against granuphilin (αGrp-N, left panel, 5-nm gold particles) and Rab27a (right panel, 10-nm gold particles). Bar, 500 nm.
FIG. 4.
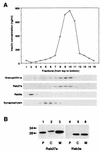
Subcellular localization of Rab27a and Rab3a. (A) A postnuclear supernatant of MIN6 cells was separated on a linear sucrose density gradient. Fifteen fractions were collected, and immunoreactive insulin in a portion of each fraction was measured (topmost panel). Equal volumes of the fractions were analyzed by immunoblotting using antibodies against granuphilin-a (79-kDa protein), Rab27a (28-kDa protein), Rab3a (26-kDa protein), and synaptophysin (36- to 38-kDa protein). (B) MIN6 cells were fractionated as described previously (5). Briefly, cells were harvested, swollen in hypotonic buffer (20 mM 3-morpholinopropanesulfonic acid [pH 7.3], 1 mM MgCl2, 0.1 mM EDTA, 1 mM phenylmethylsulfonyl fluoride), and ruptured by passage through a 26-gauge needle. After centrifugation at 100,000 × g for 60 min, the supernatant (C; soluble cytosolic fraction) was precipitated with 10% trichloroacetic acid plus 0.1% sodium deoxycholate. The pellet was resuspended in hypotonic buffer plus 1% Nonidet P-40. The suspension was centrifuged at 10,000 × g for 10 min to separate the supernatant (M; detergent-soluble membrane fraction) and the pellet (P; detergent-insoluble particulate fraction). Equal proportions of the fractions were separated on a polyacrylamide gel for immunoblotting. Numbers to the left of panel B are molecular masses in kilodaltons.
FIG. 5.
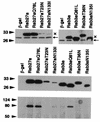
Overexpression of Rab27a and Rab3a by recombinant adenoviruses. MIN6 cells were infected 40 h before experiments with recombinant adenovirus bearing either β-Gal, Rab27a, or Rab3a cDNA. The expression levels of endogenous (←) and exogenous (×) Rab proteins were determined by immunoblotting with anti-Rab27a (upper left panel) and anti-Rab3a (upper right panel) antibodies. Note that exogenous Rab proteins migrate slower due to the Xpress tag. The expression levels of exogenous Rab27a and Rab3a were compared by immunoblotting with anti-Xpress antibodies (middle panel). Note that each form of Rab27a and Rab3a was expressed at a similar level except for Rab27a T23N and N113I. These two mutants were extremely difficult to express even by using higher PFU per cell. Granuphilin-a associated with each Rab protein was determined by immunoblotting the anti-Xpress immunoprecipitates with antigranuphilin antibodies (αGrp-aC, bottom panel). Numbers to the left of the panels are molecular masses in kilodaltons.
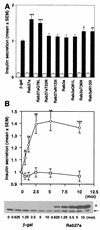
FIG. 6.
Effect of overexpression of Rab27a and Rab3a on insulin secretion. MIN6 cells were infected with recombinant adenoviruses bearing either β-Gal or Rab27a cDNA. Insulin in the medium was measured in duplicate. Values were normalized to the release of insulin from uninfected MIN6 cells stimulated by high K+. The results are given as means ± standard errors of the means (SEMs) of six (A) and five (B) independent experiments. ∗, P < 0.05; ∗∗, P < 0.005; and ∗∗∗, P < 0.0005, versus high K+-stimulated MIN6 cells infected with the same titer of the virus bearing β-Gal cDNA. (A) MIN6 cells were infected with recombinant adenoviruses under the same conditions as for Fig. 5. The cells were incubated for 30 min in either modified Krebs-Ringer buffer (open bars) or the buffer modified to include high K+ (solid bars). (B) MIN6 cells were infected with adenoviruses bearing β-Gal (□) or wild-type Rab27a cDNA (○) at different titers (multiplicity of infection, 0.625 to 10). The cells were incubated for 30 min in modified Krebs-Ringer buffer containing high K+. The expression levels of Rab27a were shown by immunoblotting with anti-Rab27a antibodies. Note that the expression levels of Rab27a with the Xpress tag (×) increased depending on the virus titer, in contrast to the constant levels of endogenous Rab27a (←).
Similar articles
- Granuphilin modulates the exocytosis of secretory granules through interaction with syntaxin 1a.
Torii S, Zhao S, Yi Z, Takeuchi T, Izumi T. Torii S, et al. Mol Cell Biol. 2002 Aug;22(15):5518-26. doi: 10.1128/MCB.22.15.5518-5526.2002. Mol Cell Biol. 2002. PMID: 12101244 Free PMC article. - Slp4-a/granuphilin-a regulates dense-core vesicle exocytosis in PC12 cells.
Fukuda M, Kanno E, Saegusa C, Ogata Y, Kuroda TS. Fukuda M, et al. J Biol Chem. 2002 Oct 18;277(42):39673-8. doi: 10.1074/jbc.M205349200. Epub 2002 Aug 9. J Biol Chem. 2002. PMID: 12176990 - The Rab27a effector exophilin7 promotes fusion of secretory granules that have not been docked to the plasma membrane.
Wang H, Ishizaki R, Xu J, Kasai K, Kobayashi E, Gomi H, Izumi T. Wang H, et al. Mol Biol Cell. 2013 Feb;24(3):319-30. doi: 10.1091/mbc.E12-04-0265. Epub 2012 Dec 5. Mol Biol Cell. 2013. PMID: 23223571 Free PMC article. - The roles of Rab27 and its effectors in the regulated secretory pathways.
Izumi T, Gomi H, Kasai K, Mizutani S, Torii S. Izumi T, et al. Cell Struct Funct. 2003 Oct;28(5):465-74. doi: 10.1247/csf.28.465. Cell Struct Funct. 2003. PMID: 14745138 Review. - [Granuphilin, a novel Rab27a effector protein, is involved in the exocytosis of dense-core granules].
Izumi T. Izumi T. Seikagaku. 2002 Apr;74(4):304-7. Seikagaku. 2002. PMID: 12030032 Review. Japanese. No abstract available.
Cited by
- Immunomodulatory effects mediated by serotonin.
Arreola R, Becerril-Villanueva E, Cruz-Fuentes C, Velasco-Velázquez MA, Garcés-Alvarez ME, Hurtado-Alvarado G, Quintero-Fabian S, Pavón L. Arreola R, et al. J Immunol Res. 2015;2015:354957. doi: 10.1155/2015/354957. Epub 2015 Apr 19. J Immunol Res. 2015. PMID: 25961058 Free PMC article. Review. - Granuphilin molecularly docks insulin granules to the fusion machinery.
Gomi H, Mizutani S, Kasai K, Itohara S, Izumi T. Gomi H, et al. J Cell Biol. 2005 Oct 10;171(1):99-109. doi: 10.1083/jcb.200505179. J Cell Biol. 2005. PMID: 16216924 Free PMC article. - Downregulation of Rab27A contributes to metformin-induced suppression of breast cancer stem cells.
Feng F, Zhang J, Fan X, Yuan F, Jiang Y, Lv R, Ma Y. Feng F, et al. Oncol Lett. 2017 Sep;14(3):2947-2953. doi: 10.3892/ol.2017.6542. Epub 2017 Jul 8. Oncol Lett. 2017. PMID: 28928832 Free PMC article. - Rab27b regulates mast cell granule dynamics and secretion.
Mizuno K, Tolmachova T, Ushakov DS, Romao M, Abrink M, Ferenczi MA, Raposo G, Seabra MC. Mizuno K, et al. Traffic. 2007 Jul;8(7):883-92. doi: 10.1111/j.1600-0854.2007.00571.x. Traffic. 2007. PMID: 17587407 Free PMC article. - The nanoscale molecular morphology of docked exocytic dense-core vesicles in neuroendocrine cells.
Prasai B, Haber GJ, Strub MP, Ahn R, Ciemniecki JA, Sochacki KA, Taraska JW. Prasai B, et al. Nat Commun. 2021 Jun 25;12(1):3970. doi: 10.1038/s41467-021-24167-9. Nat Commun. 2021. PMID: 34172739 Free PMC article.
References
- Bartel, P. L., and S. Fields. 1995. Analyzing protein-protein interactions using two-hybrid system. Methods Enzymol. 254:241-263. - PubMed
- Bock, J. B., H. T. Matern, A. A. Peden, and R. H. Scheller. 2001. A genomic perspective on membrane compartment organization. Nature 409:839-841. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Research Materials