Differential modulation of Ca(v)2.1 channels by calmodulin and Ca2+-binding protein 1 - PubMed (original) (raw)
Differential modulation of Ca(v)2.1 channels by calmodulin and Ca2+-binding protein 1
Amy Lee et al. Nat Neurosci. 2002 Mar.
Abstract
Ca(v)2.1 channels, which mediate P/Q-type Ca2+ currents, undergo Ca2+/calmodulin (CaM)-dependent inactivation and facilitation that can significantly alter synaptic efficacy. Here we report that the neuronal Ca2+-binding protein 1 (CaBP1) modulates Ca(v)2.1 channels in a manner that is markedly different from modulation by CaM. CaBP1 enhances inactivation, causes a depolarizing shift in the voltage dependence of activation, and does not support Ca2+-dependent facilitation of Ca(v)2.1 channels. These inhibitory effects of CaBP1 do not require Ca2+, but depend on the CaM-binding domain in the alpha1 subunit of Ca(v)2.1 channels (alpha12.1). CaBP1 binds to the CaM-binding domain, co-immunoprecipitates with alpha12.1 from transfected cells and brain extracts, and colocalizes with alpha12.1 in discrete microdomains of neurons in the hippocampus and cerebellum. Our results identify an interaction between Ca2+ channels and CaBP1 that may regulate Ca2+-dependent forms of synaptic plasticity by inhibiting Ca2+ influx into neurons.
Figures
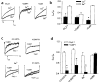
Fig. 1
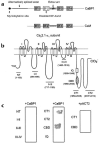
CaBP1 binds specifically to the CBD of α12.1. (a) Diagram of CaBP1 and CaM. The four Ca2+-binding EF-hand motifs are shown as boxes, and key structural differences between CaBP1 and CaM are indicated by arrows. (b) Diagram of the rat brain α12.1 subunit (rbA) showing the intracellular domains that were tested for interaction with CaBP1 in yeast two-hybrid assays. The amino acid boundaries of the indicated constructs are given in parentheses. (c) β-galactosidase assays of yeast cotransformed with the α12.1 constructs shown in (b) and either CaBP1 or control vector (pACT2).
Fig. 2
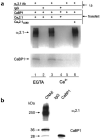
CaBP1 associates with the α12.1 subunit in tsA-201 cells and rat brain. (a) Lysates from cells transfected with Cav2.1 plus CaBP, CaBP1 alone or Cav2.1ΔCBD plus CaBP1 were subjected to immunoprecipitation (i.p.) with affinity-purified α12.1-specific antibodies or control IgG as indicated. Experiments were done with 10 mM EGTA (lanes 1 and 2) or 2 mM Ca2+ (lanes 3–6). Blots were probed with α12.1- (top) or CaBP1-specific antibodies (bottom). (b) Rat cerebellar proteins immunoprecipitated with α12.1-specific antibodies (CNA5) or control IgG were immunoblotted with α12.1- (top) or CaBP1-specific antibodies (bottom). Lysate from tsA-201 cells transfected with CaBP1 was used as a control.
Fig. 3
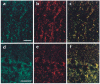
CaBP1 colocalizes with α12.1 in rat brain sections. Rat brain sections were double-labeled with antibodies specific for CaBP1 and α12.1. Labeling for CaBP1 is shown in green (a, d) and for α12.1 in red (b, e). In the merged images (c, f), double-labeled structures appear yellow. Representative examples are shown from the molecular layer of the cerebellum (a–c) and the CA1 region of the hippocampus (d–f). Scale bars, 5 μm (a–c) and 50 μm (d–f).
Fig. 4
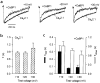
CaBP1 enhances the inactivation of _I_Ca in tsA-201 cells transfected with Cav2.1 channels. (a) Representative traces of _I_Ca from cells transfected with Cav2.1 either alone (bottom) or with CaBP1 (top). Currents were evoked by 1-s pulses to the indicated voltages from a holding potential of −80 mV and were scaled for comparison. (b) Time constants for the inactivation of Cav2.1 channel currents in the absence of CaBP1. Test currents were evoked by pulses to the indicated voltages as described in (a) and fit with a single exponential function. Data were averaged from 6–18 cells. (c) Time constants for inactivation of _I_Ca in cells cotransfected with CaBP1. Test currents were evoked by the same voltages as in (a) and (b), but current traces were fit with a double exponential function. Fast (τfast, filled bars) and slow time constants (τslow, open bars) were averaged from 7–20 cells.
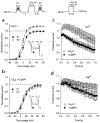
Fig. 5
Fast, Ca2+-independent inactivation of Cav2.1 channels by CaBP1 differs from the modulation of Cav2.1 channels by CaM. (a) Cav2.1 channel currents recorded with Ca2+ or Ba2+ as the permeant ion. Test pulses were applied from a holding voltage of −80 mV to +10 mV (Ca2+) or 0 mV (Ba2+) for Cav2.1 either alone or cotransfected with CaM, or to +20 mV (Ca2+) or +10 mV (Ba2+) for cells cotransfected with CaBP1, to account for the positive shift in voltage-dependent activation caused by CaBP1. The intracellular solution contained 0.5 mM EGTA. (b) The residual current amplitude at the end of a test pulse (_I_res, indicated in a) was normalized to the peak current (_I_pk) for cells transfected with Cav2.1 either alone or with CaBP1 or CaM. (c) Representative currents evoked by a test pulse to +30 mV (+20 mV for _I_Ba) in cells transfected with wild-type or mutant Cav2.1 lacking the CBD (Cav2.1ΔCBD) either alone or cotransfected with CaBP1. Intracellular solutions contained 0.5 mM EGTA except where 10 mM BAPTA is indicated and extracellular solutions contained 10 mM Ca2+ except where Ba2+ is indicated. (d) Current amplitudes at 200 ms (_I_200, indicated in c) were normalized to the peak current (_I_pk) and plotted for the different conditions. Recordings were from tsA-201 cells transfected with Cav2.1 or Cav2.1ΔCBD either alone (open bars) or with CaBP1 (filled bars). Results represent averages of 5–13 cells. Asterisks indicate statistically significant differences between the paired groups (p ≤ 0.05).
Fig. 6
CaBP1 alters the voltage dependence of Cav2.1 activation. Tail current–voltage curves from tsA-201 cells transfected with Cav2.1 (a–c) or Cav2.1ΔCBD (d) either alone (open circles) or with CaBP1 (filled circles). Test pulses (10 ms) to the indicated voltages were applied from a holding voltage of −80 mV and peak tail currents were measured upon the repolarization of cells to −40 mV, normalized to the largest tail current in the series, and plotted against test voltage. Test pulses were held for 10 ms, as activation of currents was complete but inactivation was minimal during this time. Bath solutions contained 10 mM Ca2+ (a, c, d) or Ba2+ (b), and intracellular solutions contained 0.5 mM EGTA (a, b, d) or 10 mM BAPTA (c). Each point represents the mean of 7–20 cells.
Fig. 7
CaBP1 does not support Ca2+-dependent facilitation of Cav2.1 channels. (a, b) Voltage dependence of Cav2.1 Ca2+ currents evoked before (P1, filled circles) and after (P2, open circles) a depolarizing prepulse. Tail currents were measured by repolarizing cells to −40 mV for 5 ms after variable test voltages and normalized to the largest tail current evoked by P1. Inset, representative currents evoked by a test pulse to +10 mV before (filled circles) and after (open circles) the prepulse. Intracellular recording solution contained 0.5 mM EGTA. Results were obtained from cells transfected with Cav2.1 either alone (a, n = 7) or with CaBP1 (b, n = 10). (c, d) Cav2.1 channel currents elicited by repetitive depolarizations. Test pulses (+20 mV (c) or +10 mV (d) to account for voltage shifts cause by Ba2+ substitution) at a frequency of 100 Hz were applied to cells transfected with Cav2.1 either alone (open circles) or along with CaBP1 (filled circles). Peak current amplitudes were normalized to the first pulse in the series and plotted against time during the train. Every second data point is shown. Intracellular recording solutions contained 0.5 mM EGTA, and bath solutions contained 10 mM Ca2+ (c) or Ba2+ (d). In (c), n = 9 for open circles; n = 13 for closed circles. In (d), n = 5 for open circles; n = 11 for closed circles.
Similar articles
- Differential regulation of CaV2.1 channels by calcium-binding protein 1 and visinin-like protein-2 requires N-terminal myristoylation.
Few AP, Lautermilch NJ, Westenbroek RE, Scheuer T, Catterall WA. Few AP, et al. J Neurosci. 2005 Jul 27;25(30):7071-80. doi: 10.1523/JNEUROSCI.0452-05.2005. J Neurosci. 2005. PMID: 16049184 Free PMC article. - Ca2+-binding protein-1 facilitates and forms a postsynaptic complex with Cav1.2 (L-type) Ca2+ channels.
Zhou H, Kim SA, Kirk EA, Tippens AL, Sun H, Haeseleer F, Lee A. Zhou H, et al. J Neurosci. 2004 May 12;24(19):4698-708. doi: 10.1523/JNEUROSCI.5523-03.2004. J Neurosci. 2004. PMID: 15140941 Free PMC article. - Modulation of CaV2.1 channels by the neuronal calcium-binding protein visinin-like protein-2.
Lautermilch NJ, Few AP, Scheuer T, Catterall WA. Lautermilch NJ, et al. J Neurosci. 2005 Jul 27;25(30):7062-70. doi: 10.1523/JNEUROSCI.0447-05.2005. J Neurosci. 2005. PMID: 16049183 Free PMC article. - L-Type Ca2+ Channel Regulation by Calmodulin and CaBP1.
Ames JB. Ames JB. Biomolecules. 2021 Dec 2;11(12):1811. doi: 10.3390/biom11121811. Biomolecules. 2021. PMID: 34944455 Free PMC article. Review. - The Ca2+/calmodulin system in neuronal hyperexcitability.
Solà C, Barrón S, Tusell JM, Serratosa J. Solà C, et al. Int J Biochem Cell Biol. 2001 May;33(5):439-55. doi: 10.1016/s1357-2725(01)00030-9. Int J Biochem Cell Biol. 2001. PMID: 11331200 Review.
Cited by
- Genetic polymorphism and protein conformational plasticity in the calmodulin superfamily: two ways to promote multifunctionality.
Ikura M, Ames JB. Ikura M, et al. Proc Natl Acad Sci U S A. 2006 Jan 31;103(5):1159-64. doi: 10.1073/pnas.0508640103. Epub 2006 Jan 23. Proc Natl Acad Sci U S A. 2006. PMID: 16432210 Free PMC article. - Differential regulation of CaV2.1 channels by calcium-binding protein 1 and visinin-like protein-2 requires N-terminal myristoylation.
Few AP, Lautermilch NJ, Westenbroek RE, Scheuer T, Catterall WA. Few AP, et al. J Neurosci. 2005 Jul 27;25(30):7071-80. doi: 10.1523/JNEUROSCI.0452-05.2005. J Neurosci. 2005. PMID: 16049184 Free PMC article. - Calcium binding protein-mediated regulation of voltage-gated calcium channels linked to human diseases.
Nejatbakhsh N, Feng ZP. Nejatbakhsh N, et al. Acta Pharmacol Sin. 2011 Jun;32(6):741-8. doi: 10.1038/aps.2011.64. Acta Pharmacol Sin. 2011. PMID: 21642945 Free PMC article. Review. - Control of Excitation/Inhibition Balance in a Hippocampal Circuit by Calcium Sensor Protein Regulation of Presynaptic Calcium Channels.
Nanou E, Lee A, Catterall WA. Nanou E, et al. J Neurosci. 2018 May 2;38(18):4430-4440. doi: 10.1523/JNEUROSCI.0022-18.2018. Epub 2018 Apr 13. J Neurosci. 2018. PMID: 29654190 Free PMC article. - Molecular determinants of CaV2.1 channel regulation by calcium-binding protein-1.
Few AP, Nanou E, Scheuer T, Catterall WA. Few AP, et al. J Biol Chem. 2011 Dec 9;286(49):41917-41923. doi: 10.1074/jbc.M111.292417. Epub 2011 Sep 29. J Biol Chem. 2011. PMID: 21965686 Free PMC article.
References
- Miller RJ. Multiple calcium channels and neuronal function. Science. 1987;235:46–52. - PubMed
- Catterall WA. Structure and function of neuronal Ca2+ channels and their role in neurotransmitter release. Cell Calcium. 1998;24:307–323. - PubMed
- Ikeda SR. Voltage-dependent modulation of N-type calcium channels by G-protein βγ subunits. Nature. 1996;380:255–258. - PubMed
- Herlitze S, et al. Modulation of Ca2+ channels by G protein βγ subunits. Nature. 1996;380:258–262. - PubMed
- De Waard M, et al. Direct binding of G-protein βγ complex to voltage-dependent calcium channels. Nature. 1997;385:446–450. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- R01 EY08061/EY/NEI NIH HHS/United States
- F32 NS010645/NS/NINDS NIH HHS/United States
- R01 NS22625/NS/NINDS NIH HHS/United States
- R01 EY008061/EY/NEI NIH HHS/United States
- F32 NS10645/NS/NINDS NIH HHS/United States
- R01 NS022625/NS/NINDS NIH HHS/United States
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous