Functional recovery following traumatic spinal cord injury mediated by a unique polymer scaffold seeded with neural stem cells - PubMed (original) (raw)
Functional recovery following traumatic spinal cord injury mediated by a unique polymer scaffold seeded with neural stem cells
Yang D Teng et al. Proc Natl Acad Sci U S A. 2002.
Abstract
To better direct repair following spinal cord injury (SCI), we designed an implant modeled after the intact spinal cord consisting of a multicomponent polymer scaffold seeded with neural stem cells. Implantation of the scaffold-neural stem cells unit into an adult rat hemisection model of SCI promoted long-term improvement in function (persistent for 1 year in some animals) relative to a lesion-control group. At 70 days postinjury, animals implanted with scaffold-plus-cells exhibited coordinated, weight-bearing hindlimb stepping. Histology and immunocytochemical analysis suggested that this recovery might be attributable partly to a reduction in tissue loss from secondary injury processes as well as in diminished glial scarring. Tract tracing demonstrated corticospinal tract fibers passing through the injury epicenter to the caudal cord, a phenomenon not present in untreated groups. Together with evidence of enhanced local GAP-43 expression not seen in controls, these findings suggest a possible regeneration component. These results may suggest a new approach to SCI and, more broadly, may serve as a prototype for multidisciplinary strategies against complex neurological problems.
Figures
Figure 1
(a) Schematic of the scaffold design showing the inner and outer scaffolds. (b and c) Inner scaffolds seeded with NSCs. (Scale bars: 200 μm and 50 μm, respectively.) The outer section of the scaffold was created by means of a solid–liquid phase separation technique that produced long, axially oriented pores for axonal guidance as well as radial pores to allow fluid transport and inhibit the ingrowth of scar tissue (d; scale bar, 100 μm). (e) Schematic of surgical insertion of the implant into the spinal cord.
Figure 2
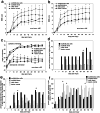
(a) BBB open-field walking scores for the four groups on the ipsilateral, lesioned side. Hindlimbs were assessed independently to determine the degree of asymmetry. The rate of improvement for the scaffold plus cells group was significantly greater than the rate for the cells-alone (P < 0.001) and lesion-control groups (P = 0.004; two-way repeated measures of ANOVA). For absolute score attained, the scaffold plus cells group showed significant improvement in open-field locomotion compared with the cells-alone (P = 0.006) and lesion-control groups (P = 0.007) for all time points from 14 days p.i. on (ANOVA, Bonferroni _post hoc_analysis). (b) BBB open-field walking scores for the contralateral unlesioned hindlimb. There is only a 1–2 point difference between the lesioned (see a) and unlesioned sides, indicating that the walking behavior of the groups was relatively symmetric overall and that both sides were impacted by this unilateral lesion. Individual animals did, however, exhibit a degree of asymmetry in their stepping with a twisted trunk position leading to the rotation of both hindlimbs to one side of their bodies. (c) Inclined plane results. When facing upwards, animals in the four groups are statistically similar for all time points (Kruskal–Wallis test) indicating that they were generally similar in strength. When facing downwards, the scaffold plus cells group performance showed statistically significant improvement on all days from day 14 on (except days 49 and 70) compared with the cells-alone and lesion-control groups (Kruskal–Wallis test). Although the data showed significant departure from the Gaussian distribution, parametric analysis revealed similar results to nonparametric analysis, and the results are represented in the figure by their means for visual clarity. (d) Righting reflex results. The scaffold plus cells group exhibited a significantly higher percentage of normal righting on day 56 as compared with the lesion-control (Pearson χ2 test of independence). (e) Percent of animals with a normal pain reflex in response to toe pinching was significantly higher in the plus cells group on the days marked by the asterisks (Pearson χ2 test). (f) Percent of animals exhibiting a spastic response to the same toe pinching stimuli.
Figure 3
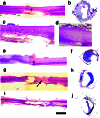
Hematoxylin/eosin (H&E) staining of representative 20-μm-thick longitudinal and transverse sections from each group. (_a_and b) Scaffold plus cells sections in longitudinal and cross section, respectively. Area with an asterisk in a_is shown at higher power in c. (a) A section from the cord of the scaffold plus cells animal from Movie 1, which is published as supporting information. Although there is some degeneration of gray matter, there is preserved tissue near the injury epicenter as well as rostral and caudal to the injury. There is only limited scar tissue at the injury epicenter on the lesioned side (c). (d_–_f) Scaffold-alone sections. Area with an asterisk in e is shown at higher power in d. (g and_h) Cells-alone sections. Note the degradation of tissue both rostral and caudal to the injury epicenter as well as the extent of scar tissue at the injury epicenter on both the lesioned and unlesioned sides (arrow). (i and j) Lesion-control sections. In i there is a small degree of white matter on the unlesioned side, but the lesioned region has expanded almost 2-fold from the original lesion size, a 4-mm lateral hemisection. In j there is an incomplete rim of peripheral white matter on the unlesioned side. (Scale bar, 2.5 mm.)
Figure 4
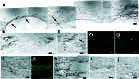
(a) BDA tracing of the corticospinal tract in a scaffold-alone animal. BDA was injected into the sensorimotor cortex contralateral to the spinal cord injury at or beyond 70 days p.i. such that BDA-positive fibers would be visible at the site of, or caudal to, the spinal cord lesion if corticospinal tract fibers were present and passing through the injury site. Arrows indicate where the tracer passes into the lesioned side of the cord. Note the tortuous path of the fibers (arrows; see text). The rostral end is to the right, and the lesion cavity is on the bottom. (b) Positive BDA tracing caudal to the injury in the same cord as in a. (c) GAP-43, a marker for regenerating axons, is positive in the same cord as seen in a and b just rostral to the injury. Likewise, scaffold plus cells cords exhibit BDA tracing with the same tortuousity immediately (d) rostral to as well as (e) caudal to the injury, and (f) a large number of GAP-43-positive axons just rostral to the injury. The cells-alone (g) and lesion-control cords (h) exhibit BDA staining rostral to the injury but not caudal, and there are minimal fibers positive for GAP-43 (i, cells alone; j, lesion control; scale bars in a_–_b,d_–_e, and_g_–h, 10 μm; in c,f, and i_–_j, 20 μm).
Figure 5
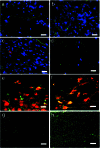
All longitudinal sections. (a) A scaffold plus cells section at the injury epicenter immunostained for NF (green) and counterstained with DAPI (blue). (b) Scaffold-alone section at the injury epicenter exhibiting NF immunoreactivity (green) within the region excised by the initial lesion, suggesting that the new tissue contains neurons or neuronal elements (blue, DAPI). (c) Double immunostaining for NF (green) and the mouse-specific antigen M2 used to identify donor cells (red) in a scaffold plus cells cord and DAPI (blue). The cord has extensive NF immunopositivity, but there is no clear colocalization of NF and M2, suggesting that the NF+ cells were of host-origin. (d) There are few NF+ cells near the injury epicenter in the lesion-control cord. (e) Scaffold plus cells section doubled-immunostained for GFAP (green) and M2 (red). The majority of donor-derived cells (red) are not double-labeled for GFAP, suggesting that they do not contribute to the astroglial scar. (f) Most donor cells (stained with the M2 antibody, green in this figure) are double-labeled for nestin (red in this figure), as seen by confocal microscopy. GFAP immunostaining (green) in the spared tissue contralateral to the hemisection in a scaffold plus cells cord (g) compared with a lesion-control cord (h). There is a far greater incidence of astrocytes at the injury epicenter of the lesion-control compared with the scaffold-alone cord. (Scale bars:a_–_f, 20 μm;g_–_h, 10 μm.)
Similar articles
- Brain and spinal cord injury repair by implantation of human neural progenitor cells seeded onto polymer scaffolds.
Shin JE, Jung K, Kim M, Hwang K, Lee H, Kim IS, Lee BH, Lee IS, Park KI. Shin JE, et al. Exp Mol Med. 2018 Apr 20;50(4):1-18. doi: 10.1038/s12276-018-0054-9. Exp Mol Med. 2018. PMID: 29674624 Free PMC article. - Embryonic intermediate filament, nestin, expression following traumatic spinal cord injury in adult rats.
Shibuya S, Miyamoto O, Auer RN, Itano T, Mori S, Norimatsu H. Shibuya S, et al. Neuroscience. 2002;114(4):905-16. doi: 10.1016/s0306-4522(02)00323-8. Neuroscience. 2002. PMID: 12379246 - Transplants and neurotrophic factors increase regeneration and recovery of function after spinal cord injury.
Bregman BS, Coumans JV, Dai HN, Kuhn PL, Lynskey J, McAtee M, Sandhu F. Bregman BS, et al. Prog Brain Res. 2002;137:257-73. doi: 10.1016/s0079-6123(02)37020-1. Prog Brain Res. 2002. PMID: 12440372 Review.
Cited by
- The use of poly(N-[2-hydroxypropyl]-methacrylamide) hydrogel to repair a T10 spinal cord hemisection in rat: a behavioural, electrophysiological and anatomical examination.
Pertici V, Amendola J, Laurin J, Gigmes D, Madaschi L, Carelli S, Marqueste T, Gorio A, Decherchi P. Pertici V, et al. ASN Neuro. 2013 May 30;5(2):149-66. doi: 10.1042/AN20120082. ASN Neuro. 2013. PMID: 23614684 Free PMC article. - Synergistic effects of Buyang Huanwu decoction and embryonic neural stem cell transplantation on the recovery of neurological function in a rat model of spinal cord injury.
Zhang M, Chai Y, Liu T, Xu N, Yang C. Zhang M, et al. Exp Ther Med. 2015 Apr;9(4):1141-1148. doi: 10.3892/etm.2015.2248. Epub 2015 Feb 2. Exp Ther Med. 2015. PMID: 25780400 Free PMC article. - 3D Printed Stem-Cell Derived Neural Progenitors Generate Spinal Cord Scaffolds.
Joung D, Truong V, Neitzke CC, Guo SZ, Walsh PJ, Monat JR, Meng F, Park SH, Dutton JR, Parr AM, McAlpine MC. Joung D, et al. Adv Funct Mater. 2018 Sep 26;28(39):1801850. doi: 10.1002/adfm.201801850. Epub 2018 Aug 9. Adv Funct Mater. 2018. PMID: 32595422 Free PMC article. - Host reaction to poly(2-hydroxyethyl methacrylate) scaffolds in a small spinal cord injury model.
Li HY, Führmann T, Zhou Y, Dalton PD. Li HY, et al. J Mater Sci Mater Med. 2013 Aug;24(8):2001-11. doi: 10.1007/s10856-013-4956-8. Epub 2013 May 24. J Mater Sci Mater Med. 2013. PMID: 23702616 - Biomaterial design strategies for the treatment of spinal cord injuries.
Straley KS, Foo CW, Heilshorn SC. Straley KS, et al. J Neurotrauma. 2010 Jan;27(1):1-19. doi: 10.1089/neu.2009.0948. J Neurotrauma. 2010. PMID: 19698073 Free PMC article. Review.
References
- Beattie M S, Farooqui A A, Bresnahan J C. J Neurotrauma. 2000;17:915–925. - PubMed
- Chen M S, Huber A B, Haar M E v d, Frank M, Schnell L, Spillmann A A, Christ F, Schwab M E. Nature (London) 2000;403:434–439. - PubMed
- Davies S J, Fitch M T, Memberg S P, Hall A K, Raisman G, Silver J. Nature (London) 1997;390:680–683. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous