The human pathogen Pseudomonas aeruginosa utilizes conserved virulence pathways to infect the social amoeba Dictyostelium discoideum - PubMed (original) (raw)
The human pathogen Pseudomonas aeruginosa utilizes conserved virulence pathways to infect the social amoeba Dictyostelium discoideum
Stefan Pukatzki et al. Proc Natl Acad Sci U S A. 2002.
Abstract
Genetically accessible host models are useful for studying microbial pathogenesis because they offer the means to identify novel strategies that pathogens use to evade immune mechanisms, cause cellular injury, and induce disease. We have developed conditions under which the human pathogen Pseudomonas aeruginosa infects Dictyostelium discoideum, a genetically tractable eukaryotic organism. When D. discoideum is plated on nutrient agar plates with different P. aeruginosa strains, the bacteria form lawns on these plates with amoebae embedded in them. Virulent P. aeruginosa strains kill these amoebae and leave an intact bacterial lawn. A number of P. aeruginosa mutants have been identified that are avirulent in this assay. Amoebae feed on these bacteria and form plaques in their bacterial lawns. One avirulent mutant strain carries an insertional mutation in the lasR gene. LasR is a transcription factor that controls a number of virulence genes in a density-dependent fashion. Another class of avirulent P. aeruginosa mutants is defective in type III secretion. One mutant lacks the PscJ protein, a structural component of the secretion apparatus, suggesting that cytotoxins are injected into the D. discoideum cell. One of these cytotoxins is ExoU, and exoU mutants are avirulent toward D. discoideum. Complementation of the lasR and exoU mutations restores virulence. Therefore, P. aeruginosa uses conserved virulence pathways to kill D. discoideum.
Figures
Figure 1
D. discoideum cells do not form plaques in lawns of_P. aeruginosa_ strain PA14. D. discoideum_cells (AX3) were plated on SM/5 with K. aerogenes(Left) and P. aeruginosa strains PA14 (Right) at a density of ≈100 D. discoideum cells/plate. After 3 days, D. discoideum plaques appeared on plates with K. aerogenes. No plaques were formed on plates with the virulent_P. aeruginosa strain.
Figure 2
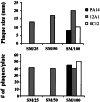
Complementation of the lasR mutation restores LasR function and virulence but does not affect synthesis of the pigment pyocyanin. (Top) D. discoideum cells (AX3) were plated on nutrient agar (SM/5) with P. aeruginosa strain PA14, the isogenic lasR_-mutant 12A1, complemented strains SUP16 (Δ_lasR with pUCP18) and SUP17 (Δ_lasR_ with plasR-PUH), and the pyocyanin-deficient strain ΔphenA/ΔphenB. Plates were incubated at 22°C for 5 days, and virulence was assessed by determining the number of Dictyostelium_plaques. (Middle) Elastase activity of supernatants from overnight cultures were assayed by using elastin Congo red (ECR) as the substrate. Data represent the means of duplicate elastase assays, and activity is expressed as the activity relative to wild-type PA14 (=100%) +/−SD_n−1 .(Bottom) The ability of each strain to secrete the pigment pyocyanin was determined by assaying the amount of pyocyanin present in overnight cultures of each strain. Pyocyanin was extracted from supernatants and assayed as described in (20). Data represent the means of duplicate assays, and amounts of secreted pyocyanin are expressed as micrograms/milliliter culture medium +/− SD_n−1_.
Figure 3
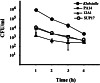
Uptake of P. aeruginosa by D. discoideum.D. discoideum cells were placed in tissue culture wells at 106 cells/ml. Cells were infected with bacteria at a multiplicity of infection of approximately 100:1. Cultures were incubated at 22°C for 30 min, at which time gentamicin was added to kill all extracellular bacteria. Amoebae were collected at indicated time points, lysed, and plated on nutrient agar plates to determine the colony-forming units (cfu) in these lysates. Data represent the means of duplicate experiments, and the number of internalized bacteria per well is expressed as cfu/ml +/− SD_n−1_.
Figure 4
D. discoideum forms plaques in thin lawns of P. aeruginosa PA14. D. discoideum cells (AX3) were plated with P. aeruginosa strains PA14 (wild type) and PA14 mutants 8C12 and 12A1 (ΔlasR) on nutrient agar plates with decreasing nutrient content. Agar plates contained 1/25 (SM/25), 1/50 (SM/50), and 1/100 (SM/100) of the nutrient content in SM plates. After 4 days at 22°C, D. discoideum formed plaques in lawns of 12A1 on SM/25 and plates with lower nutrient content, 8C12 and PA14 allowed plaque formation only on SM/100 plates.
Figure 5
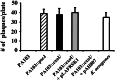
Complementation of the exoU mutation restores virulence.D. discoideum cells (AX3) were plated with different bacterial associates on nutrient agar plates (SM/5) at a density of approximately 40 amoebae/plate. Emerging D. discoideum plaques on each plate were scored after 5 days. Virulence is expressed as the average number of emerging plaques per plate, and error bars represent the standard deviation of five replicates. The virulence of PA103, as well as isogenic mutants PA103∷pscJ (Δ_pscJ_), PA103∷exoU (Δ_exoU_), PA103∷exoU+pLAFRSKI (Δ_exoU_ with plasmid control pLAFRSKI), and PA103∷exoU+pAH807 [Δ_exoU_ with plasmid pAH807 (exoU+/spcU+)] was tested in this assay. As a reference for plating efficiency, AX3 was also cultured with_K. aerogenes_.
Figure 6
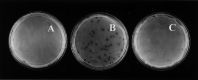
Expression of ExoU in P. aeruginosa PA103 prohibits plaque formation by D. discoideum. D. discoideum cells (AX3) were plated on SM/5 with_P. aeruginosa_ strains PA103 (A), PA103∷exoU (Δ_exoU_) (B), and PA103∷exoU + pAH807 (Δ_exoU_ with plasmid pAH807 [exoU+/spcU+]) (C) at a density of ≈100 _D. discoideum_cells/plate. Plaques were scored after 4 days of incubation at 22°C.
Similar articles
- Assessing Pseudomonas aeruginosa virulence using a nonmammalian host: Dictyostelium discoideum.
Filion G, Charette SJ. Filion G, et al. Methods Mol Biol. 2014;1149:671-80. doi: 10.1007/978-1-4939-0473-0_51. Methods Mol Biol. 2014. PMID: 24818941 - Pseudomonas aeruginosa virulence analyzed in a Dictyostelium discoideum host system.
Cosson P, Zulianello L, Join-Lambert O, Faurisson F, Gebbie L, Benghezal M, Van Delden C, Curty LK, Köhler T. Cosson P, et al. J Bacteriol. 2002 Jun;184(11):3027-33. doi: 10.1128/JB.184.11.3027-3033.2002. J Bacteriol. 2002. PMID: 12003944 Free PMC article. - Evolution of Pseudomonas aeruginosa virulence in infected patients revealed in a Dictyostelium discoideum host model.
Lelong E, Marchetti A, Simon M, Burns JL, van Delden C, Köhler T, Cosson P. Lelong E, et al. Clin Microbiol Infect. 2011 Sep;17(9):1415-20. doi: 10.1111/j.1469-0691.2010.03431.x. Epub 2011 Feb 3. Clin Microbiol Infect. 2011. PMID: 21091830 - Dictyostelium as host model for pathogenesis.
Steinert M, Heuner K. Steinert M, et al. Cell Microbiol. 2005 Mar;7(3):307-14. doi: 10.1111/j.1462-5822.2005.00493.x. Cell Microbiol. 2005. PMID: 15679834 Review. - Eat, kill or die: when amoeba meets bacteria.
Cosson P, Soldati T. Cosson P, et al. Curr Opin Microbiol. 2008 Jun;11(3):271-6. doi: 10.1016/j.mib.2008.05.005. Epub 2008 Jun 10. Curr Opin Microbiol. 2008. PMID: 18550419 Review.
Cited by
- Alternative host model to evaluate Aeromonas virulence.
Froquet R, Cherix N, Burr SE, Frey J, Vilches S, Tomas JM, Cosson P. Froquet R, et al. Appl Environ Microbiol. 2007 Sep;73(17):5657-9. doi: 10.1128/AEM.00908-07. Epub 2007 Jul 6. Appl Environ Microbiol. 2007. PMID: 17616616 Free PMC article. - The Pseudomonas aeruginosa accessory genome elements influence virulence towards Caenorhabditis elegans.
Vasquez-Rifo A, Veksler-Lublinsky I, Cheng Z, Ausubel FM, Ambros V. Vasquez-Rifo A, et al. Genome Biol. 2019 Dec 10;20(1):270. doi: 10.1186/s13059-019-1890-1. Genome Biol. 2019. PMID: 31823826 Free PMC article. - STING mediates immune responses in the closest living relatives of animals.
Woznica A, Kumar A, Sturge CR, Xing C, King N, Pfeiffer JK. Woznica A, et al. Elife. 2021 Nov 3;10:e70436. doi: 10.7554/eLife.70436. Elife. 2021. PMID: 34730512 Free PMC article. - Biomedical applications of genome-scale metabolic network reconstructions of human pathogens.
Dunphy LJ, Papin JA. Dunphy LJ, et al. Curr Opin Biotechnol. 2018 Jun;51:70-79. doi: 10.1016/j.copbio.2017.11.014. Epub 2017 Dec 7. Curr Opin Biotechnol. 2018. PMID: 29223465 Free PMC article. Review. - The rise of pathogens: predation as a factor driving the evolution of human pathogens in the environment.
Erken M, Lutz C, McDougald D. Erken M, et al. Microb Ecol. 2013 May;65(4):860-8. doi: 10.1007/s00248-013-0189-0. Epub 2013 Jan 27. Microb Ecol. 2013. PMID: 23354181 Free PMC article. Review.
References
- Deretic V. In: Persistent Bacterial Infections. Nataro J P, Blaser M J, Cunningham-Rundles S, editors. Washington, DC: Am. Soc. Microbiol.; 2000. pp. 305–326.
- Singh P K, Schaefer A L, Parsek M R, Moninger T O, Welsh M J, Greenberg E P. Nature (London) 2000;407:762–764. - PubMed
- Mahajan-Miklos S, Rahme L G, Ausubel F M. Mol Microbiol. 2000;37:981–988. - PubMed
- Kessin R H. Dictyostelium—Evolution, Cell Biology, and the Development of Multicellularity. Cambridge, U.K.: Cambridge Univ. Press; 2001.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources