An extracellular matrix-associated zinc metalloprotease is required for dilauroyl phosphatidylethanolamine chemotactic excitation in Myxococcus xanthus - PubMed (original) (raw)
Comparative Study
An extracellular matrix-associated zinc metalloprotease is required for dilauroyl phosphatidylethanolamine chemotactic excitation in Myxococcus xanthus
Daniel B Kearns et al. J Bacteriol. 2002 Mar.
Abstract
An extracellular matrix connects bacteria that live in organized assemblages called biofilms. While the role of the matrix in the regulation of cell behavior has not been extensively examined in bacteria, we suggest that, like mammalian cells, the matrix facilitates cell-cell interactions involved with regulation of cohesion, motility, and sensory transduction. The extracellular matrix of the soil bacterium Myxococcus xanthus is essential for biofilm formation and fruiting body development. The matrix material is extruded as long, thin fibrils that mediate adhesion to surfaces, cohesion to other cells, and excitation by the chemoattractant dilauroyl phosphatidylethanolamine. We report the identification of a putative matrix-associated zinc metalloprotease called FibA (fibril protein A). Western blotting with FibA-specific monoclonal antibody 2105 suggests extensive proteolytic processing of FibA during assembly into fibrils, consistent with the autoprocessing observed with other members of the M4 metalloprotease family. Disruption of fibA had no obvious effect on the structure of the fibrils and did not inhibit cell cohesion, excitation by dioleoyl phosphatidylethanolamine, or activity of the A- or S-motility motors. However, the cells lost the ability to respond to dilauroyl phosphatidylethanolamine and to form well-spaced fruiting bodies, though substantial aggregation was observed. Chemotactic excitation of the fibA mutant was restored by incubation with purified wild-type fibrils. The results suggest that this metalloprotease is involved in sensory transduction.
Figures
FIG. 1.
Amino acid sequence alignment of M. xanthus FibA with the AhpB elastase of A. hydrophila (accession number AAF07184) and LasB elastase of P. aeruginosa (accession number AAG07111). Open circles mark the FibA putative active site residues. The putative nucleotide binding P-loop is marked with closed circles. The putative type II secretion signal sequence is underlined with a solid black bar, while the Behmlander and Dworkin (5) amino acid sequence determined by N-terminal sequencing is underlined with a gray bar.
FIG. 2.
The fibA mutant is proficient in fibril biosynthesis. Scanning electron micrographs of DK1622 wild type (a) and LS2200 fibA::pDB23 (b) are shown. Cells were incubated for 30 min in cohesion buffer prior to fixation. Bar = 1 μm.
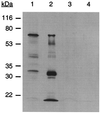
FIG. 3.
The fibA mutant lacks all MAb 2105 reactive polypeptides. A Western blot with MAb 2105 as primary antibody is shown. Lane 1, lysate from 5 × 108 whole cells of wild-type DK1622; lane 2, 5 μg of protein from purified DK1622 fibrils; lane 3, lysate from 5 × 108 whole cells of LS2200 (fibA); lane 4, 5 μg of protein from purified LS2200 fibrils.
FIG. 4.
fibA produces elongated fruiting bodies. Wild-type (a) and fibA::pDB23 LS2200 (b) cells (5 × 107) were starved on TPM agar for 5 days to induce fruiting body formation. Bar = 2 mm.
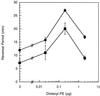
FIG. 5.
fibA has normal excitation to dioleoyl PE. The reversal periods of fibA::pDB23 LS2200 (circles) and the wild type (DK1622) (squares). Bars show the standard deviations of three replicates. The wild-type data are derived from that of Kearns et al. (22).
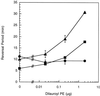
FIG. 6.
fibA is deficient in excitation to dilauroyl PE. The reversal periods of fibA::pDB23 LS2200 (circles), the wild type (DK1622) (squares), and LS2200 cells incubated with purified wild-type fibrils (triangles). Bars show the standard deviations of three replicates.
Similar articles
- FibA and PilA act cooperatively during fruiting body formation of Myxococcus xanthus.
Bonner PJ, Black WP, Yang Z, Shimkets LJ. Bonner PJ, et al. Mol Microbiol. 2006 Sep;61(5):1283-93. doi: 10.1111/j.1365-2958.2006.05298.x. Mol Microbiol. 2006. PMID: 16925559 - Myxococcus xanthus fibril appendages are essential for excitation by a phospholipid attractant.
Kearns DB, Campbell BD, Shimkets LJ. Kearns DB, et al. Proc Natl Acad Sci U S A. 2000 Oct 10;97(21):11505-10. doi: 10.1073/pnas.210448597. Proc Natl Acad Sci U S A. 2000. PMID: 11016978 Free PMC article. - Lipid chemotaxis and signal transduction in Myxococcus xanthus.
Kearns DB, Shimkets LJ. Kearns DB, et al. Trends Microbiol. 2001 Mar;9(3):126-9. doi: 10.1016/s0966-842x(01)01948-5. Trends Microbiol. 2001. PMID: 11239790 Review. - The Dif chemosensory pathway is directly involved in phosphatidylethanolamine sensory transduction in Myxococcus xanthus.
Bonner PJ, Xu Q, Black WP, Li Z, Yang Z, Shimkets LJ. Bonner PJ, et al. Mol Microbiol. 2005 Sep;57(5):1499-508. doi: 10.1111/j.1365-2958.2005.04785.x. Mol Microbiol. 2005. PMID: 16102016 - Gliding motility in bacteria: insights from studies of Myxococcus xanthus.
Spormann AM. Spormann AM. Microbiol Mol Biol Rev. 1999 Sep;63(3):621-41. doi: 10.1128/MMBR.63.3.621-641.1999. Microbiol Mol Biol Rev. 1999. PMID: 10477310 Free PMC article. Review.
Cited by
- Interaction between extracellular lipase LipA and the polysaccharide alginate of Pseudomonas aeruginosa.
Tielen P, Kuhn H, Rosenau F, Jaeger KE, Flemming HC, Wingender J. Tielen P, et al. BMC Microbiol. 2013 Jul 13;13:159. doi: 10.1186/1471-2180-13-159. BMC Microbiol. 2013. PMID: 23848942 Free PMC article. - Identification and localization of Myxococcus xanthus porins and lipoproteins.
Bhat S, Zhu X, Patel RP, Orlando R, Shimkets LJ. Bhat S, et al. PLoS One. 2011;6(11):e27475. doi: 10.1371/journal.pone.0027475. Epub 2011 Nov 22. PLoS One. 2011. PMID: 22132103 Free PMC article. - Cohesion-defective mutants of Myxococcus xanthus.
Bonner PJ, Shimkets LJ. Bonner PJ, et al. J Bacteriol. 2006 Jun;188(12):4585-8. doi: 10.1128/JB.00237-06. J Bacteriol. 2006. PMID: 16740967 Free PMC article. - A CheW homologue is required for Myxococcus xanthus fruiting body development, social gliding motility, and fibril biogenesis.
Bellenger K, Ma X, Shi W, Yang Z. Bellenger K, et al. J Bacteriol. 2002 Oct;184(20):5654-60. doi: 10.1128/JB.184.20.5654-5660.2002. J Bacteriol. 2002. PMID: 12270823 Free PMC article. - Reprolysin metalloproteases from Ixodes persulcatus, Rhipicephalus sanguineus and Rhipicephalus microplus ticks.
Ali A, Tirloni L, Isezaki M, Seixas A, Konnai S, Ohashi K, da Silva Vaz Junior I, Termignoni C. Ali A, et al. Exp Appl Acarol. 2014 Aug;63(4):559-78. doi: 10.1007/s10493-014-9796-9. Epub 2014 Apr 1. Exp Appl Acarol. 2014. PMID: 24687173
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources