The circadian regulatory proteins BMAL1 and cryptochromes are substrates of casein kinase Iepsilon - PubMed (original) (raw)
The circadian regulatory proteins BMAL1 and cryptochromes are substrates of casein kinase Iepsilon
Erik J Eide et al. J Biol Chem. 2002.
Abstract
The serine/threonine protein kinase casein kinase I epsilon (CKIepsilon) is a key regulator of metazoan circadian rhythm. Genetic and biochemical data suggest that CKIepsilon binds to and phosphorylates the PERIOD proteins. However, the PERIOD proteins interact with a variety of circadian regulators, suggesting the possibility that CKIepsilon may interact with and phosphorylate additional clock components as well. We find that CRY1 and BMAL1 are phosphoproteins in cultured cells. Mammalian PERIOD proteins act as a scaffold with distinct domains that simultaneously bind CKIepsilon and mCRY1 and mCRY2 (mCRY). mCRY is phosphorylated by CKIepsilon only when both proteins are bound to mammalian PERIOD proteins. BMAL1 is also a substrate for CKIepsilon in vitro, and CKIepsilon kinase activity positively regulates BMAL1-dependent transcription from circadian promoters in reporter assays. We conclude that CKIepsilon phosphorylates multiple circadian substrates and may exert its effects on circadian rhythm in part by a direct effect on BMAL1-dependent transcription.
Figures
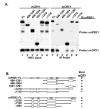
Fig. 1. mCRY interacts with a conserved domain in the carboxyl terminus of mPER1 and mPER2
A, HEK 293 cells were transiently transfected with plasmids expressing full-length V5 epitope-tagged mCRY1 and the indicated truncations of Myc epitope-tagged mPER1 or mPER2. mPER was immunoprecipitated (IP) with anti-Myc antibodies and the presence of mCRY in the immunoprecipitate was assessed by immunoblotting with anti-V5 antibodies. B, schematic representation of mCRY·mPER binding results, with selected domains on mPER indicated. Boxes labeled A and B indicate PAS domains, whereas CKIε indicates the CKIε/δ binding site, and N indicates one of the nuclear localization signals. FL, full length.
Fig. 2. CKIε interaction with mCRY1 requires intact PER protein
Endogenous CKIε co-immunoprecipitates (IP) with mCRY1 only in the presence of full-length mPER proteins. Myc epitope-tagged mCRY1 and FLAG epitope-tagged mPER were transiently expressed in HEK 293 cells as indicated. mCRY was immunoprecipitated with anti-Myc antibodies, and the presence of co-precipitating mPER was assessed by SDS-PAGE and immunoblotting with anti FLAG antibodies. The blot was sequentially stripped and reprobed with an anti-CKIε polyclonal antibody to detect the presence of co-immunoprecipitating endogenous CKIε and with an anti-Myc antibody to confirm the presence of mCRY1 in the immunoprecipitate pellet. An equal amount of input cell lysates is shown on the left (lanes 1–5), whereas the proteins present in the immunoprecipitate are shown on the right (_lanes 1_′–_5_′).
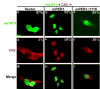
Fig. 3. mCRY1 promotes nuclear localization of the mPER1/CKIε complex
CKIε is localized to the nucleus through interaction with the PER·CRY complex. Myc-tagged mCRY1 and hemagglutinin epitope-tagged CKIε were transiently expressed in HEK 293 cells in the presence of vector (a, d, and g), full-length mPER1 (b, e, and h), or truncated mPER1 (Δ1118, lacking the CRY interaction domain) (c, f, and i) as indicated. Protein localization was visualized by staining the cells with anti-Myc antibodies conjugated to Alexa 488 (green) and anti-hemagglutinin antibodies conjugated to Alexa 594 (red). The percent of transfected cells exhibiting nuclear localization of the indicated protein is shown in each panel.
Fig. 4. mPER-dependent mCRY1 phosphorylation
A, mCRY1 is a phosphoprotein in intact cells. HEK 293 cells were transiently transfected with either empty vector (−) or with a plasmid encoding Myc-tagged mCRY1 (+). 18 h after transfection, cells were metabolically labeled with [32P]orthophosphoric acid for 3 h, at which time cell free lysates were prepared and subjected to immunoprecipitation with anti-Myc antibody. 32P incorporation into mCRY1 was assessed by SDS-PAGE and phosphorimaging analysis. B, mCRY1 is phosphorylated by CKIε in a CKIε· PER·mCRY1 complex in vitro. HEK 293 cells were transiently transfected with mCRY1 and PER expression plasmids as indicated. mCRY1 protein was then immunoprecipitated (IP) with anti-Myc antibodies, and the immunoprecipitates were split three ways. Two of the three fractions were subjected to an in vitro kinase assay in the presence of [γ-32P]ATP and without (−) or with (+) added recombinant CKIε (ΔC320). mCRY1 phosphorylation was then visualized by SDS-PAGE and phosphorimaging. C, the third bead fraction was subjected to immunoblotting after SDS-PAGE to confirm the presence of CRY and PER proteins in the immunoprecipitates as indicated in the figure.
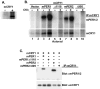
Fig. 5. BMAL1 phosphorylation is regulated by CKIε in vitro and in intact cells
A, BMAL1 but not CLK is a substrate of CKI. V5 epitope-tagged CLK (lanes 1, 3, and 4) and BMAL1 (lanes 2, 5, and 6) were expressed in rabbit reticulocyte lysates and immunoprecipitated, and the beads were split three ways. One fraction was probed with anti-V5 antibodies to verify the presence of protein in the pellet (lanes 1 and 2), and the remaining fractions were subjected to an in vitro kinase reaction in the absence (lanes 3 and 5) or presence (lanes 4 and 6) of added recombinant CKIε (ΔC320) as indicated. B, BMAL1 was phosphorylated by CKIε in vitro independent of mPER2. HEK 293 cells were transiently transfected with plasmids encoding Myc-tagged BMAL1 along with FLAG-tagged mPER2 or empty vector. The Myc-tagged BMAL1 was immunoprecipitated and incubated with [γ-32P]ATP and without (−) or with (+) recombinant CKIε (ΔC320). Co-immunoprecipitation of mPER2 with BMAL1 was confirmed by immunoblotting a fraction of the immunoprecipitates with anti-FLAG antibodies (lanes 5 and 6). The presence of co-immunoprecipitating mPER2 did not stimulate phosphorylation of BMAL1. C, BMAL phosphorylation in intact cells is reduced by co-expression of dominant-negative CKIε. HEK 293 cells were transiently transfected with plasmids encoding Myc epitope-tagged BMAL1 and either vector (−) or a kinase-inactive form of CKIε (K38A) (+). At 18 h post-transfection, cells were labeled with [32P]orthophosphoric acid for 3 h, and then the BMAL1 protein was immunoprecipitated (IP) from lysates with anti-Myc monoclonal antibodies. 32P incorporation into BMAL1 was assessed by SDS-PAGE and phosphorimaging analysis. BMAL1 protein expression was determined by immunoblot of cell free lysate. The data shown is a representative result of three separate experiments. D, Quantitation of BMAL1 phosphorylation without (−) or with (+) co-expression of CKIε (K38A). The results shown are the average of three independent experiments.
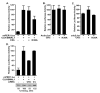
Fig. 6. CKIε regulates BMAL1 driven transcription
A, overexpression of dominant-negative CKIε leads to decreased BMAL1-driven transcription. HEK 293 cells were transiently transfected with a CLK·BMAL1-responsive promoter (mPer1:luc) and CLK, BMAL1, and CKIε expression constructs or empty vector as indicated. After 18 h, cell free extracts were prepared, and the luciferase activity was analyzed. B and C, CKIε (K38A) has minimal effect on other promoters. HEK 293 cells were transiently transfected with plasmids encoding either CKIε or dominant-negative CKIε (K38A) and a reporter with the actin promoter (B) or the cdc2 promoter (C) driving luciferase expression. 18 h after transfection, luciferase activity was analyzed as in A. D, dsRNA-mediated interference leads to depletion of CKIε/δ and inhibition of CLK/BMAL1-driven gene expression. Endogenous CKIε/δ was partially eliminated in HEK 293 cells by transfection of a 21-nucleotide RNA duplex (siRNA) directed against the CKIε/δ sequence. As a control, the sequence was introduced in the inverted orientation (Inv). After 48 h, the transfection mixture was removed, and plasmids encoding both CLK and BMAL1 (where indicated) along with a reporter containing luciferase behind the mPer1 promoter (18) were introduced. After an additional 24 h, whole cell lysates were prepared, and luciferase activity analyzed as described in A. The lower panel shows a representative immunoblot of CKIε. The relative amount of endogenous CKIε was analyzed using NIH Image software and the quantitation shown beneath.
Similar articles
- Control of intracellular dynamics of mammalian period proteins by casein kinase I epsilon (CKIepsilon) and CKIdelta in cultured cells.
Akashi M, Tsuchiya Y, Yoshino T, Nishida E. Akashi M, et al. Mol Cell Biol. 2002 Mar;22(6):1693-703. doi: 10.1128/MCB.22.6.1693-1703.2002. Mol Cell Biol. 2002. PMID: 11865049 Free PMC article. - mCRY1 and mCRY2 are essential components of the negative limb of the circadian clock feedback loop.
Kume K, Zylka MJ, Sriram S, Shearman LP, Weaver DR, Jin X, Maywood ES, Hastings MH, Reppert SM. Kume K, et al. Cell. 1999 Jul 23;98(2):193-205. doi: 10.1016/s0092-8674(00)81014-4. Cell. 1999. PMID: 10428031 - A role for cryptochromes in sleep regulation.
Wisor JP, O'Hara BF, Terao A, Selby CP, Kilduff TS, Sancar A, Edgar DM, Franken P. Wisor JP, et al. BMC Neurosci. 2002 Dec 20;3:20. doi: 10.1186/1471-2202-3-20. Epub 2002 Dec 20. BMC Neurosci. 2002. PMID: 12495442 Free PMC article. - Casein kinase I: another cog in the circadian clockworks.
Eide EJ, Virshup DM. Eide EJ, et al. Chronobiol Int. 2001 May;18(3):389-98. doi: 10.1081/cbi-100103963. Chronobiol Int. 2001. PMID: 11475410 Review. - [Genetic regulation of circadian rhythms].
Tei H. Tei H. Tanpakushitsu Kakusan Koso. 2004 Feb;49(3 Suppl):456-62. Tanpakushitsu Kakusan Koso. 2004. PMID: 14976772 Review. Japanese. No abstract available.
Cited by
- Control of mammalian circadian rhythm by CKIepsilon-regulated proteasome-mediated PER2 degradation.
Eide EJ, Woolf MF, Kang H, Woolf P, Hurst W, Camacho F, Vielhaber EL, Giovanni A, Virshup DM. Eide EJ, et al. Mol Cell Biol. 2005 Apr;25(7):2795-807. doi: 10.1128/MCB.25.7.2795-2807.2005. Mol Cell Biol. 2005. PMID: 15767683 Free PMC article. - A detailed predictive model of the mammalian circadian clock.
Forger DB, Peskin CS. Forger DB, et al. Proc Natl Acad Sci U S A. 2003 Dec 9;100(25):14806-11. doi: 10.1073/pnas.2036281100. Epub 2003 Dec 1. Proc Natl Acad Sci U S A. 2003. PMID: 14657377 Free PMC article. - Inhibition of casein kinase I epsilon/delta produces phase shifts in the circadian rhythms of Cynomolgus monkeys.
Sprouse J, Reynolds L, Swanson TA, Engwall M. Sprouse J, et al. Psychopharmacology (Berl). 2009 Jul;204(4):735-42. doi: 10.1007/s00213-009-1503-x. Epub 2009 Mar 11. Psychopharmacology (Berl). 2009. PMID: 19277609 - Probing the relative importance of molecular oscillations in the circadian clock.
Zheng X, Sehgal A. Zheng X, et al. Genetics. 2008 Mar;178(3):1147-55. doi: 10.1534/genetics.107.088658. Genetics. 2008. PMID: 18385110 Free PMC article. Review. - The tight junction protein TJP1 regulates the feeding-modulated hepatic circadian clock.
Liu Y, Zhang Y, Li T, Han J, Wang Y. Liu Y, et al. Nat Commun. 2020 Jan 30;11(1):589. doi: 10.1038/s41467-020-14470-2. Nat Commun. 2020. PMID: 32001717 Free PMC article.
References
- Eide EJ, Virshup DM. Chronobiol Int. 2001;18:389 –398. - PubMed
- Reppert SM, Weaver DR. Annu Rev Physiol. 2001;63:647–676. - PubMed
- Lowrey PL, Takahashi JS. Annu Rev Genet. 2000;34:533–562. - PubMed
- Allada R, Emery P, Takahashi JS, Rosbash M. Annu Rev Neurosci. 2001;24:1091–1119. - PubMed
- Kloss B, Price JL, Saez L, Blau J, Rothenfluh A, Wesley CS, Young MW. Cell. 1998;94:97–107. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases