Functional neurogenesis in the adult hippocampus - PubMed (original) (raw)
Functional neurogenesis in the adult hippocampus
Henriette van Praag et al. Nature. 2002.
Abstract
There is extensive evidence indicating that new neurons are generated in the dentate gyrus of the adult mammalian hippocampus, a region of the brain that is important for learning and memory. However, it is not known whether these new neurons become functional, as the methods used to study adult neurogenesis are limited to fixed tissue. We use here a retroviral vector expressing green fluorescent protein that only labels dividing cells, and that can be visualized in live hippocampal slices. We report that newly generated cells in the adult mouse hippocampus have neuronal morphology and can display passive membrane properties, action potentials and functional synaptic inputs similar to those found in mature dentate granule cells. Our findings demonstrate that newly generated cells mature into functional neurons in the adult mammalian brain.
Conflict of interest statement
Gompeting interests statement
The authors declare that they have no competing financial interests.
Figures
Figure 1
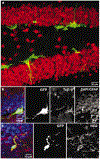
Dividing cells in the adult mouse dentate gyrus express early neural markers at 48 h after virus injection. a, Confocal micrograph (a merged image of 15 1-µm optical sections) of GFP expression in the dentate gyrus in a section that was labelled for the neuronal marker NeuN (red). No co-labelling of GFP+ cells with NeuN was observed. b, Single confocal plane of a cluster of GFP+ cells labelled with the early neuronal marker Tuj1-β (red). c, Single confocal plane of a GFP+ cell with immunoreactivity for the progenitor marker NG2 (red). In b and c, nuclei (DAPI) and GFAP are blue.
Figure 2
GFP+ cells in the dentate gyrus 4 weeks after virus injection express mature neuronal markers. a, Overview of GFP expression in the dentate gyrus in a section (a merged image of 27 1-µm optical sections) that was labelled for the neuronal marker NeuN (red). b, GFP+ cell (a merged image of 18 1-µm optical sections) immunoreactive for NeuN. c, Single confocal plane of the same cell, showing co-localization of GFP and NeuN.
Figure 3
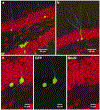
Newly generated neurons receive synaptic inputs. a, Confocal photomicrograph (a merged image of 10 0.5-µm optical sections) of a GFP+ cell, immunolabelled for calbindin (blue) and synaptophysin (red). Adjacent panels (merged images of 4 0.5-µm optical sections) show co-localization with calbindin and synaptophysin. b–e, Visualization of spines in GFP+ cells (merged images of 12–33 optical sections of 0.5 µm). The boxed areas in the insets correspond to the enlarged images of the dendrites. Cells were analysed 4 months (b, c) or 4 weeks (d, e) after virus injection. f, Electron micrograph of synaptic terminals (arrows) on the soma of a GFP+ neuron (asterisk) in the granule cell layer.
Figure 4
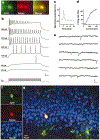
Newly generated cells display neuronal electrophysiological properties. a, Fluorescent micrographs of a GFP+ cell filled with Alexa through a recording pipette. b, Membrane potential in response to depolarizing currents (_I_m; 500 ms, 20–400 pA) recorded under current clamp at the resting potential (−73.5 mV). Numbers on the left indicate stimulus size. Scale: 25 mV, 50 ms. c, Action potential recorded at 80 pA. d, Firing rate versus current curve. e, Spontaneous postsynaptic currents recorded under voltage clamp (−80 mV). Scale: 20 pA, 100 ms. f, Confocal micrographs taken from a single plane (1 µm) after fixation of the slice. g, Overview of the dorsal blade of the granule cell layer (merged z-series of 23 planes).
Figure 5
Newly generated neurons are functionally similar to mature dentate granule cells. Consecutive recordings of a GFP+ neuron (right) and an adjacent granule cell (left) are shown. The GFP+ neuron (_C_m = 32 pF) was smaller and, therefore, younger than the unlabelled neuron (_C_m = 60 pF). a, Fluorescence micrographs of both cells filled with Alexa (left panels). The right panel is a confocal micrograph taken after fixation, with GFP (green), Alexa (red) and DAPI (blue) visualized. b, Membrane potential in response to depolarizing current steps. Stimulus size is indicated on the left. Scale: 50 mV, 50 ms. c, Postsynaptic currents evoked by paired-pulse stimulation (100 µA, 0.2 ms, with a 50-ms interval) of the perforant path. The position of the stimulating electrode was identical for both neurons. Transients before the traces are stimulus artifacts. Scale: 100 pA, 25 ms (left); 40 pA, 25 ms (right). d, Spiking rate versus current curve. Insets show a single action potential.
Similar articles
- Similar GABAergic inputs in dentate granule cells born during embryonic and adult neurogenesis.
Laplagne DA, Kamienkowski JE, Espósito MS, Piatti VC, Zhao C, Gage FH, Schinder AF. Laplagne DA, et al. Eur J Neurosci. 2007 May;25(10):2973-81. doi: 10.1111/j.1460-9568.2007.05549.x. Epub 2007 May 17. Eur J Neurosci. 2007. PMID: 17509085 - Neurogenesis after transient global ischemia in the adult hippocampus visualized by improved retroviral vector.
Tanaka R, Yamashiro K, Mochizuki H, Cho N, Onodera M, Mizuno Y, Urabe T. Tanaka R, et al. Stroke. 2004 Jun;35(6):1454-9. doi: 10.1161/01.STR.0000126480.40967.b3. Epub 2004 Apr 8. Stroke. 2004. PMID: 15073392 - Adult neurogenesis in the mammalian dentate gyrus.
Abbott LC, Nigussie F. Abbott LC, et al. Anat Histol Embryol. 2020 Jan;49(1):3-16. doi: 10.1111/ahe.12496. Epub 2019 Sep 30. Anat Histol Embryol. 2020. PMID: 31568602 Review. - Preferential Targeting of Lateral Entorhinal Inputs onto Newly Integrated Granule Cells.
Woods NI, Vaaga CE, Chatzi C, Adelson JD, Collie MF, Perederiy JV, Tovar KR, Westbrook GL. Woods NI, et al. J Neurosci. 2018 Jun 27;38(26):5843-5853. doi: 10.1523/JNEUROSCI.1737-17.2018. Epub 2018 May 23. J Neurosci. 2018. PMID: 29793975 Free PMC article. - Functional circuits of new neurons in the dentate gyrus.
Vivar C, van Praag H. Vivar C, et al. Front Neural Circuits. 2013 Feb 25;7:15. doi: 10.3389/fncir.2013.00015. eCollection 2013. Front Neural Circuits. 2013. PMID: 23443839 Free PMC article. Review.
Cited by
- Toxicity of the flame-retardant BDE-49 on brain mitochondria and neuronal progenitor striatal cells enhanced by a PTEN-deficient background.
Napoli E, Hung C, Wong S, Giulivi C. Napoli E, et al. Toxicol Sci. 2013 Mar;132(1):196-210. doi: 10.1093/toxsci/kfs339. Epub 2013 Jan 3. Toxicol Sci. 2013. PMID: 23288049 Free PMC article. - Effects of Microglia on Neurogenesis.
Sato K. Sato K. Glia. 2015 Aug;63(8):1394-405. doi: 10.1002/glia.22858. Epub 2015 May 24. Glia. 2015. PMID: 26010551 Free PMC article. Review. - MicroRNA-124 and -137 cooperativity controls caspase-3 activity through BCL2L13 in hippocampal neural stem cells.
Schouten M, Fratantoni SA, Hubens CJ, Piersma SR, Pham TV, Bielefeld P, Voskuyl RA, Lucassen PJ, Jimenez CR, Fitzsimons CP. Schouten M, et al. Sci Rep. 2015 Jul 24;5:12448. doi: 10.1038/srep12448. Sci Rep. 2015. PMID: 26207921 Free PMC article. - N,N-dimethyltryptamine compound found in the hallucinogenic tea ayahuasca, regulates adult neurogenesis in vitro and in vivo.
Morales-Garcia JA, Calleja-Conde J, Lopez-Moreno JA, Alonso-Gil S, Sanz-SanCristobal M, Riba J, Perez-Castillo A. Morales-Garcia JA, et al. Transl Psychiatry. 2020 Sep 28;10(1):331. doi: 10.1038/s41398-020-01011-0. Transl Psychiatry. 2020. PMID: 32989216 Free PMC article. - Recruitment of parvalbumin and somatostatin interneuron inputs to adult born dentate granule neurons.
Remmers CL, Castillon CCM, Armstrong JN, Contractor A. Remmers CL, et al. Sci Rep. 2020 Oct 16;10(1):17522. doi: 10.1038/s41598-020-74385-2. Sci Rep. 2020. PMID: 33067500 Free PMC article.
References
- Altman J & Das GD Autoradiographic and histological evidence of postnatal neurogenesis in rats. J. Comp. Neurol l24, 319–335 (1965). - PubMed
- Kaplan MS & Hinds JW Neurogenesis in the adult rat: electron microscopic analysis of light radioautographs. Science l97, 1092–1094 (1977). - PubMed
- Eriksson PS et al. Neurogenesis in the adult human hippocampus. Nature Med 4, 1313–1317 (1998). - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources