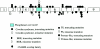Protean PTEN: form and function - PubMed (original) (raw)
Review
Protean PTEN: form and function
Kristin A Waite et al. Am J Hum Genet. 2002 Apr.
Abstract
Germline mutations distributed across the PTEN tumor-suppressor gene have been found to result in a wide spectrum of phenotypic features. Originally shown to be a major susceptibility gene for both Cowden syndrome (CS), which is characterized by multiple hamartomas and an increased risk of breast, thyroid, and endometrial cancers, and Bannayan-Riley-Ruvalcaba syndrome, which is characterized by lipomatosis, macrocephaly, and speckled penis, the PTEN hamartoma tumor syndrome spectrum has broadened to include Proteus syndrome and Proteus-like syndromes. Exon 5, which encodes the core motif, is a hotspot for mutations likely due to the biology of the protein. PTEN is a major lipid 3-phosphatase, which signals down the PI3 kinase/AKT pro-apoptotic pathway. Furthermore, PTEN is a protein phosphatase, with the ability to dephosphorylate both serine and threonine residues. The protein-phosphatase activity has also been shown to regulate various cell-survival pathways, such as the mitogen-activated kinase (MAPK) pathway. Although it is well established that PTEN's lipid-phosphatase activity, via the PI3K/AKT pathway, mediates growth suppression, there is accumulating evidence that the protein-phosphatase/MAPK pathway is equally important in the mediation of growth arrest and other crucial cellular functions.
Figures
Figure 1
Germline PTEN mutations in CS, BRRS, PS, and Proteus-like syndromes
Figure 2
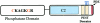
Protein domains of PTEN. The N-terminal phosphatase domain (amino acids 1–185) is shown with the catalytic core. The missense mutations which have been crucial for the elucidation of the cellular role of PTEN are highlighted in orange. Mutations at C124 render a lipid- and protein-phosphatase–inactive protein, whereas mutations at G129 result in a lipid-phosphatase–inactive yet protein-phosphatase–active PTEN. The C-terminal domain (amino acids 186–403) contains the lipid-binding C2 domain (amino acids 186–351); PEST domains (amino acids 350–375 and 379–396), which regulate protein stability; and the PDZ domain, which is important in protein-protein interactions. The CK2 phosphorylation sites (S380, T382, and T383), which are important for stability, are indicated by the blue asterisks (*).
Figure 3
PTEN as a regulator of the PI3K pathway. Ligand binding to membrane receptors results in the activation of PI3K and the subsequent increase in PIP3, which recruits PDK1 to the cellular membrane. PDK1 phosphorylates and activates AKT, which in turn regulates a variety of cellular processes. PTEN dephosphorylates PI3P, lowering its cellular levels and resulting in the down-regulation of AKT.
Figure 4
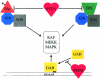
PTEN as a modulator of the MAPK pathway. PTEN can inhibit the activation of MAPK by several mechanisms. By dephosphorylating Shc and/or IRS-1, PTEN prevents the association of these proteins to the Sos:Grb complex, which is required for MAPK activation. Gab interacts with the membrane by binding to PI3P regions via the pleckstrin-homology domain. By decreasing the PI3P levels in the membrane, PTEN inhibits the translocation of Gab to the membrane and its subsequent activation of the MAPK pathways. The entire pathway for MAPK activation and protein-protein interactions has been omitted for clarity.
Similar articles
- PTEN: one gene, many syndromes.
Eng C. Eng C. Hum Mutat. 2003 Sep;22(3):183-98. doi: 10.1002/humu.10257. Hum Mutat. 2003. PMID: 12938083 Review. - Association of germline mutation in the PTEN tumour suppressor gene and Proteus and Proteus-like syndromes.
Zhou X, Hampel H, Thiele H, Gorlin RJ, Hennekam RC, Parisi M, Winter RM, Eng C. Zhou X, et al. Lancet. 2001 Jul 21;358(9277):210-1. doi: 10.1016/s0140-6736(01)05412-5. Lancet. 2001. PMID: 11476841 - Germline PTEN promoter mutations and deletions in Cowden/Bannayan-Riley-Ruvalcaba syndrome result in aberrant PTEN protein and dysregulation of the phosphoinositol-3-kinase/Akt pathway.
Zhou XP, Waite KA, Pilarski R, Hampel H, Fernandez MJ, Bos C, Dasouki M, Feldman GL, Greenberg LA, Ivanovich J, Matloff E, Patterson A, Pierpont ME, Russo D, Nassif NT, Eng C. Zhou XP, et al. Am J Hum Genet. 2003 Aug;73(2):404-11. doi: 10.1086/377109. Epub 2003 Jul 3. Am J Hum Genet. 2003. PMID: 12844284 Free PMC article. - Germline and germline mosaic PTEN mutations associated with a Proteus-like syndrome of hemihypertrophy, lower limb asymmetry, arteriovenous malformations and lipomatosis.
Zhou XP, Marsh DJ, Hampel H, Mulliken JB, Gimm O, Eng C. Zhou XP, et al. Hum Mol Genet. 2000 Mar 22;9(5):765-8. doi: 10.1093/hmg/9.5.765. Hum Mol Genet. 2000. PMID: 10749983 - Role of PTEN, a lipid phosphatase upstream effector of protein kinase B, in epithelial thyroid carcinogenesis.
Eng C. Eng C. Ann N Y Acad Sci. 2002 Jun;968:213-21. doi: 10.1111/j.1749-6632.2002.tb04337.x. Ann N Y Acad Sci. 2002. PMID: 12119278 Review.
Cited by
- Phosphatase and tensin homologue (PTEN) regulates synaptic plasticity independently of its effect on neuronal morphology and migration.
Sperow M, Berry RB, Bayazitov IT, Zhu G, Baker SJ, Zakharenko SS. Sperow M, et al. J Physiol. 2012 Feb 15;590(4):777-92. doi: 10.1113/jphysiol.2011.220236. Epub 2011 Dec 6. J Physiol. 2012. PMID: 22147265 Free PMC article. - Polyp Genetics.
Klos CL, Dharmarajan S. Klos CL, et al. Clin Colon Rectal Surg. 2016 Dec;29(4):289-295. doi: 10.1055/s-0036-1582442. Clin Colon Rectal Surg. 2016. PMID: 31777459 Free PMC article. Review. - PTEN inhibits adrenomedullin expression and function in brain tumor cells.
Betchen SA, Musatov S, Roberts J, Pena J, Kaplitt MG. Betchen SA, et al. J Neurooncol. 2006 Sep;79(2):117-23. doi: 10.1007/s11060-005-9035-7. Epub 2006 Jul 5. J Neurooncol. 2006. PMID: 16821090 - Dysregulation of mTOR signaling in fragile X syndrome.
Sharma A, Hoeffer CA, Takayasu Y, Miyawaki T, McBride SM, Klann E, Zukin RS. Sharma A, et al. J Neurosci. 2010 Jan 13;30(2):694-702. doi: 10.1523/JNEUROSCI.3696-09.2010. J Neurosci. 2010. PMID: 20071534 Free PMC article. - Downregulation of PTEN at corneal wound sites accelerates wound healing through increased cell migration.
Cao L, Graue-Hernandez EO, Tran V, Reid B, Pu J, Mannis MJ, Zhao M. Cao L, et al. Invest Ophthalmol Vis Sci. 2011 Apr 8;52(5):2272-8. doi: 10.1167/iovs.10-5972. Print 2011 Apr. Invest Ophthalmol Vis Sci. 2011. PMID: 21212174 Free PMC article.
References
Electronic-Database Information
- Online Mendelian Inheritance in Man (OMIM), http://www.ncbi.nlm.nih.gov/Omim/ (for Charcot-Marie-Tooth syndrome [MIM 118200], BRRS [MIM 153480], CS [MIM 158350], JPS [MIM 174900], PJS [MIM 175200], PS [MIM 176920], congenital hypomyelinating neuropathy [MIM 605253], and PTEN/MMAC1/TEP1 [MIM 601728])
References
- Ahmed SF, Marsh DJ, Weremowicz S, Morton CC, Williams DM, Eng C (1999) Balanced translocation of 10q and 13q, including the PTEN gene, in a boy with an HCG-secreting tumor and the Bannayan-Riley-Ruvalcaba syndrome. J Clin Endocrinol Metab 84:4665–4670 - PubMed
- Arch EM, Goodman BK, van Wesep RA, Liaw D, Clarke K, Parsons R, McKusick VA, Geraghty MT (1997) Deletion of PTEN in a patient with Bannayan-Riley-Ruvalcaba syndrome suggests allelism with Cowden disease. Am J Med Genet 71:489–493 - PubMed
- Biesecker LG, Happle R, Mulliken JB, Weksberg R, Graham JM, Viljoen DL, Cohen MM (1999) Proteus syndrome: diagnostic criteria, differential diagnosis and patient evaluation. Am J Med Genet 84:389–395 - PubMed
- Bonneau D, Longy M (2000) Mutations of the human PTEN gene. Hum Mutat 16:109–122 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials